Ferroptosis: mechanisms and therapeutic targets
- PMID: 39568772
- PMCID: PMC11577302
- DOI: 10.1002/mco2.70010
Ferroptosis: mechanisms and therapeutic targets
Abstract
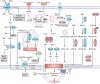
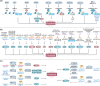
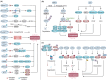
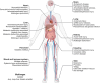
Ferroptosis is a nonapoptotic form of cell death characterized by iron-dependent lipid peroxidation in membrane phospholipids. Since its identification in 2012, extensive research has unveiled its involvement in the pathophysiology of numerous diseases, including cancers, neurodegenerative disorders, organ injuries, infectious diseases, autoimmune conditions, metabolic disorders, and skin diseases. Oxidizable lipids, overload iron, and compromised antioxidant systems are known as critical prerequisites for driving overwhelming lipid peroxidation, ultimately leading to plasma membrane rupture and ferroptotic cell death. However, the precise regulatory networks governing ferroptosis and ferroptosis-targeted therapy in these diseases remain largely undefined, hindering the development of pharmacological agonists and antagonists. In this review, we first elucidate core mechanisms of ferroptosis and summarize its epigenetic modifications (e.g., histone modifications, DNA methylation, noncoding RNAs, and N6-methyladenosine modification) and nonepigenetic modifications (e.g., genetic mutations, transcriptional regulation, and posttranslational modifications). We then discuss the association between ferroptosis and disease pathogenesis and explore therapeutic approaches for targeting ferroptosis. We also introduce potential clinical monitoring strategies for ferroptosis. Finally, we put forward several unresolved issues in which progress is needed to better understand ferroptosis. We hope this review will offer promise for the clinical application of ferroptosis-targeted therapies in the context of human health and disease.
Keywords: epigenetics; ferroptosis; human disease; lipid peroxidation.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
References
-
- Dixon SJ, Olzmann JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25(6):424‐442. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
