Challenges in institutional ethical review process and approval for international multicenter clinical studies in lower and middle-income countries: the case of PARITY study
- PMID: 39568786
- PMCID: PMC11577162
- DOI: 10.3389/fped.2024.1460377
Challenges in institutional ethical review process and approval for international multicenter clinical studies in lower and middle-income countries: the case of PARITY study
Abstract
Background: One of the greatest challenges to conducting multicenter research studies in low and middle-income countries (LMICs) is the heterogeneity in regulatory processes across sites. Previous studies have reported variations in requirements with a lack of standardization in the Institutional Review Board (IRB) processes between centers, imposing barriers for approval, participation, and development of multicenter research.
Objectives: To describe the regulatory process, variability and challenges faced by pediatric researchers in LMICs during the IRB process of an international multicenter observational point prevalence study (Global PARITY).
Design: A 16-question multiple-choice online survey was sent to site principal investigators (PIs) at PARITY study participating centers to explore characteristics of the IRB process, costs, and barriers to research approval. A shorter survey was employed for sites that expressed interest in participating in Global PARITY and started the approval process, but ultimately did not participate in data collection (non-participating sites) to assess IRB characteristics.
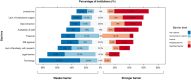
Results: Of the 91 sites that sought IRB approval, 46 were successful in obtaining approval and finishing the data collection process. The survey was completed by 46 (100%) participating centers and 21 (47%) non-participating centers. There was a significant difference between participating and non-participating sites in IRB approval of a waiver consent and in the requirement for a legal review of the protocol. The greatest challenge to research identified by non-participating sites was a lack of research time and the lack of institutional support.
Conclusions: Global collaborative research is crucial to increase our understanding of pediatric critical care conditions in hospitals of all resource-levels and IRBs are required to ensure that this research complies with ethical standards. Critical barriers restrict research activities in some resource limiting countries. Increasing the efficiency and accessibility of local IRB review could greatly impact participation of resource limited sites and enrollment of vulnerable populations.
Keywords: IRBs; Institutional Review Boards; challenges; ethics; global; low- and middle-income countries; research.
© 2024 Lopez-Baron, Abbas, Caporal, Agulnik, Attebery, Holloway, Kissoon, Mulgado-Aguas, Amegan-Aho, Majdalani, Ocampo, Pascal, Miller, Kanyamuhunga, Tekleab, Bacha, González-Dambrauskas, Bhutta, Kortz, Murthy, Remy and the Global Health Subgroup of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources