A potent and selective anti-glutathione peroxidase 4 nanobody as a ferroptosis inducer
- PMID: 39568902
- PMCID: PMC11575642
- DOI: 10.1039/d4sc05448b
A potent and selective anti-glutathione peroxidase 4 nanobody as a ferroptosis inducer
Abstract
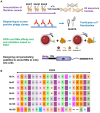
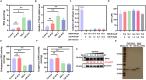
Glutathione peroxidase 4 (GPX4) plays a crucial role in the ferroptosis pathway, emerging as a potential drug target in the treatment of refractory tumors. Unfortunately, the development of GPX4-targeted treatment has been very limited due to the poor selectivity and drug-like properties of current GPX4 inhibitors. Here, we report a proof-of-concept study of potent anti-GPX4 nanobodies, successfully identified through immunizing Bactrian camels and constructing a phage library. Utilizing a cell-penetrating peptide fusion strategy, these nanobodies with high affinities to GPX4 efficiently internalized in cells and formed the basis for further applications. In particular, 12E significantly inhibited cellular GPX4 and consequently induced remarkable ferroptosis in cancer cells. Furthermore, 12E could impair zebrafish dorsal organizer formation in vivo, as evidenced by a phenotype comparable to that observed in zebrafish with the gpx4b gene knocked out. The new GPX4-inhibiting nanobody described here exhibits superior proteome-wide selectivity and a vastly improved safety profile compared to existing GPX4 inhibitors. These incredible features of 12E, as an anti-GPX4 nanobody, may not only contribute to ferroptosis-related anticancer treatment but also establish a new paradigm for nanobodies in drug development for traditionally undruggable targets.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
X. L., Y. L., Z. X., Y. W., and X. Q. are inventors of patent applications related to this work.
Figures




Similar articles
-
Glutathione peroxidase 4 inhibits Wnt/β-catenin signaling and regulates dorsal organizer formation in zebrafish embryos.Development. 2017 May 1;144(9):1687-1697. doi: 10.1242/dev.144261. Epub 2017 Mar 16. Development. 2017. PMID: 28302747 Free PMC article.
-
Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles.Nat Chem Biol. 2020 May;16(5):497-506. doi: 10.1038/s41589-020-0501-5. Epub 2020 Mar 30. Nat Chem Biol. 2020. PMID: 32231343 Free PMC article.
-
GPX4-AUTAC induces ferroptosis in breast cancer by promoting the selective autophagic degradation of GPX4 mediated by TRAF6-p62.Cell Death Differ. 2025 May 20. doi: 10.1038/s41418-025-01528-1. Online ahead of print. Cell Death Differ. 2025. PMID: 40394165
-
Recent Progress of Glutathione Peroxidase 4 Inhibitors in Cancer Therapy.Mini Rev Med Chem. 2025;25(1):42-57. doi: 10.2174/0113895575308546240607073310. Mini Rev Med Chem. 2025. PMID: 38879766 Review.
-
Prospects for Anti-Tumor Mechanism and Potential Clinical Application Based on Glutathione Peroxidase 4 Mediated Ferroptosis.Int J Mol Sci. 2023 Jan 13;24(2):1607. doi: 10.3390/ijms24021607. Int J Mol Sci. 2023. PMID: 36675129 Free PMC article. Review.
Cited by
-
The Different Cellular Entry Routes for Drug Delivery Using Cell Penetrating Peptides.Biol Cell. 2025 Jun;117(6):e70012. doi: 10.1111/boc.70012. Biol Cell. 2025. PMID: 40490965 Free PMC article. Review.
References
-
- Viswanathan V. S. Ryan M. J. Dhruv H. D. Gill S. Eichhoff O. M. Seashore-Ludlow B. Kaffenberger S. D. Eaton J. K. Shimada K. Aguirre A. J. et al., Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547(7664):453–457. doi: 10.1038/nature23007. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

