Phenacylselenoesters allow facile selenium transfer and hydrogen selenide generation
- PMID: 39568918
- PMCID: PMC11575540
- DOI: 10.1039/d4sc05788k
Phenacylselenoesters allow facile selenium transfer and hydrogen selenide generation
Abstract
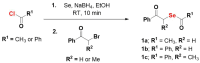
Hydrogen selenide (H2Se) is a precursor to several selenium-containing biomolecules and is emerging as an important redox-active species in biology, with yet to be completely characterized roles. Tools that reliably generate H2Se are key to achieving a better understanding of selenium biology. Here, we report the design, synthesis and evaluation of phenacylselenoesters as sources of H2Se. These compounds are prepared in two steps from commercial compounds, some are crystalline solids, and all are stable during storage. In the presence of esterase and a thiol in pH 7.4 buffer, these compounds produce H2Se, with half-lives of 5-20 min. We developed a colorimetric assay for the detection of gaseous H2Se by trapping it as zinc selenide (ZnSe), which is then converted to lead selenide (PbSe), which serves as a convenient visual indicator for this gas. The major organic products that are formed in nearly quantitative yields are relatively benign ketones and carboxylic acids. We provide evidence for these donors producing a thioselenide, a key intermediate in biological selenium metabolism. Finally, we compared sulfur and selenium transfer, both critical processes in cells. Phenacylthiol is relatively stable to cleavage by a thiol, and requires a sulfurtransferase enzyme to produce a persulfide and H2S. By contrast, the selenium analogue reacted with a thiol in the absence of this enzyme to produce H2Se. This result underscores the greater lability of the C-Se bond as compared with a C-S bond, and may have implications in biological selenium transfer. Together, phenacylselenoesters are easy to prepare, stable and generate H2Se under mild and biocompatible conditions. We anticipate that these will be valuable additions to the growing selenium redox toolbox.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Cysteine-Activated Small-Molecule H2Se Donors Inspired by Synthetic H2S Donors.J Am Chem Soc. 2022 Mar 9;144(9):3957-3967. doi: 10.1021/jacs.1c12006. Epub 2022 Feb 22. J Am Chem Soc. 2022. PMID: 35192764
-
An Examination of Chemical Tools for Hydrogen Selenide Donation and Detection.Molecules. 2024 Aug 15;29(16):3863. doi: 10.3390/molecules29163863. Molecules. 2024. PMID: 39202942 Free PMC article. Review.
-
Direct hydrogen selenide (H2Se) release from activatable selenocarbamates.Chem Sci. 2023 Jun 20;14(27):7581-7588. doi: 10.1039/d3sc01936e. eCollection 2023 Jul 12. Chem Sci. 2023. PMID: 37449078 Free PMC article.
-
Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donors for Intracellular H2Se Delivery.J Am Chem Soc. 2021 Nov 24;143(46):19542-19550. doi: 10.1021/jacs.1c09525. Epub 2021 Nov 9. J Am Chem Soc. 2021. PMID: 34752701
-
New Insights into Oxidative and Reductive Stress Responses and Their Relation to the Anticancer Activity of Selenium-Containing Compounds as Hydrogen Selenide Donors.Biology (Basel). 2023 Jun 17;12(6):875. doi: 10.3390/biology12060875. Biology (Basel). 2023. PMID: 37372159 Free PMC article. Review.
References
-
- Saito Y., Redox Experimental Medicine, 2022, R149–R158
LinkOut - more resources
Full Text Sources

