Unified enantiospecific synthesis of drimane meroterpenoids enabled by enzyme catalysis and transition metal catalysis
- PMID: 39568920
- PMCID: PMC11575645
- DOI: 10.1039/d4sc06060a
Unified enantiospecific synthesis of drimane meroterpenoids enabled by enzyme catalysis and transition metal catalysis
Abstract
Merging the advantages of biocatalysis and chemocatalysis in retrosynthetic analysis can significantly improve the efficiency and selectivity of natural product synthesis. Here, we describe a unified approach for the synthesis of drimane meroterpenoids by combining heterologous biosynthesis, enzymatic hydroxylation, and transition metal catalysis. In phase one, drimenol was produced by engineering a biosynthetic pathway in Escherichia coli. Cytochrome P450BM3 from Bacillus megaterium was engineered to catalyze the C-3 hydroxylation of drimenol. By means of nickel-catalyzed reductive coupling, six drimane meroterpenoids (+)-hongoquercins A and B, (+)-ent-chromazonarol, 8-epi-puupehenol, (-)-pelorol, and (-)-mycoleptodiscin A were synthesized in a concise and enantiospecific manner. This strategy offers facile access to the congeners of the drimane meroterpenoid family and lays the foundation for activity optimization.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

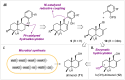



References
-
- Corey E. J. and Cheng X.-M., The Logic of Chemical Synthesis, Wiley, 1995
- Warren S. and Wyatt P., Organic Synthesis: The Disconnection Approach, Wiley, 2nd edn, 2008
-
- Brill Z. G. Condakes M. L. Ting C. P. Maimone T. J. Chem. Rev. 2017;117:11753–11795. doi: 10.1021/acs.chemrev.6b00834. - DOI - PMC - PubMed
- Urabe D. Asaba T. Inoue M. Chem. Rev. 2015;115:9207–9231. doi: 10.1021/cr500716f. - DOI - PubMed
- Nicolaou K. C. Edmonds D. J. Bulger P. G. Angew. Chem., Int. Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. - DOI - PubMed
- Wilson R. M. Danishefsky S. J. J. Org. Chem. 2007;72:4293–4305. doi: 10.1021/jo070871s. - DOI - PubMed
-
- Bornscheuer U. T. Huisman G. W. Kazlauskas R. J. Lutz S. Moore J. C. Robins K. Nature. 2012;485:185–194. doi: 10.1038/nature11117. - DOI - PubMed
- Devine P. N. Howard R. M. Kumar R. Thompson M. P. Truppo M. D. Turner N. J. Nat. Rev. Chem. 2018;2:409–421. doi: 10.1038/s41570-018-0055-1. - DOI
- Simić S. Zukić E. Schmermund L. Faber K. Winkler C. K. Kroutil W. Chem. Rev. 2022;122:1052–1126. doi: 10.1021/acs.chemrev.1c00574. - DOI - PubMed
- France S. P. Lewis R. D. Martinez C. A. JACS Au. 2023;3:715–735. doi: 10.1021/jacsau.2c00712. - DOI - PMC - PubMed
-
- Turner N. J. O'Reilly E. Nat. Chem. Biol. 2013;9:285–288. doi: 10.1038/nchembio.1235. - DOI - PubMed
- Hönig M. Sondermann P. Turner N. J. Carreira E. M. Angew. Chem., Int. Ed. 2017;56:8942–8973. doi: 10.1002/anie.201612462. - DOI - PubMed
- de Souza R. O. M. A. Miranda L. S. M. Bornscheuer U. T. Chem.–Eur. J. 2017;23:12040–12063. doi: 10.1002/chem.201702235. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

