Cyclo[4]pyrrole with α- β direct linkages
- PMID: 39568921
- PMCID: PMC11575546
- DOI: 10.1039/d4sc06670g
Cyclo[4]pyrrole with α- β direct linkages
Abstract
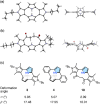
A cyclo[4]pyrrole bearing pyrrole C(α)-C(β) direct linkages, a contracted porphyrin analogue with no meso-carbon bridge, was synthesized from an oligoketone-related precursor. X-ray crystallography and StrainViz analysis revealed a non-planar structure with a total strain of 20.8 kcal mol-1. The cyclo[4]pyrrole emits fluorescence in the visible region with a quantum yield of 0.026. The NICS calculations indicated a local 6π-aromatic character for each pyrrole unit, and the global π-electronic communication among the four pyrrole units was shown by the frontier orbitals in the neutral form. The cyclo[4]pyrrole underwent reversible stepwise electrochemical two-electron oxidation and the spin density of the radical cation intermediate localized on the 3,2':5',3''-terpyrrole moiety. However, spectroelectrochemical measurements and theoretical calculations indicated the contribution of a triplet diradical dication form with the spin density delocalized across the four pyrrole units and smaller dihedral angles between neighboring aromatic rings. The structural and electrochemical behavior of cyclo[4]pyrrole demonstrated the effects of ring contraction and recombination of the pyrrole-pyrrole linkages on the cyclo[n]pyrrole system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

