Differentiating carrier protein interactions in biosynthetic pathways using dapoxyl solvatochromism
- PMID: 39568935
- PMCID: PMC11575542
- DOI: 10.1039/d4sc05499g
Differentiating carrier protein interactions in biosynthetic pathways using dapoxyl solvatochromism
Abstract
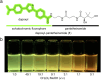
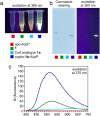
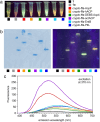
Carrier protein-dependent synthases are ubiquitous enzymes involved both in primary and secondary metabolism. Biocatalysis within these synthases is governed by key interactions between the carrier protein, substrate, and partner enzymes. The weak and transient nature of these interactions has rendered them difficult to study. Here we develop a useful fluorescent solvatochromic probe, dapoxyl-pantetheinamide, to monitor and quantify carrier protein interactions in vitro. Upon loading with target carrier proteins, we observe dramatic shifts in fluorescence emission wavelength and intensity and further demonstrate that this tool has the potential to be applied across numerous biosynthetic pathways. The environmental sensitivity of this probe allows rapid characterization of carrier protein interactions, with the ability to quantitatively determine inhibition of protein-protein interactions. We anticipate future application of these probes for inhibitor screening and in vivo characterization.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Regional cerebral blood flow single photon emission computed tomography for detection of Frontotemporal dementia in people with suspected dementia.Cochrane Database Syst Rev. 2015 Jun 23;2015(6):CD010896. doi: 10.1002/14651858.CD010896.pub2. Cochrane Database Syst Rev. 2015. PMID: 26102272 Free PMC article.
-
Why Are Autistic People More Likely to Experience Suicidal Thoughts? Applying the Integrated Motivational-Volitional Model with Autistic Adults.Autism Adulthood. 2024 Sep 16;6(3):272-283. doi: 10.1089/aut.2023.0039. eCollection 2024 Sep. Autism Adulthood. 2024. PMID: 39371353 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.

