Wiring proton gradients for energy conversion
- PMID: 39568944
- PMCID: PMC11575586
- DOI: 10.1039/d4sc04833d
Wiring proton gradients for energy conversion
Abstract
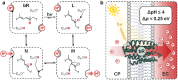
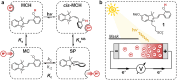
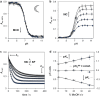
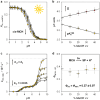
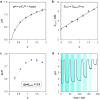
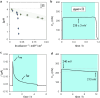
Light-switchable buffer solutions based on merocyanine photoacids can be used as efficient photoenergy harvesting systems. Varying the solvation environment of merocyanine photoacids in water-methanol mixtures allows one to carefully tune their photoacidity, relaxation kinetics, and solubility, opening up the possibility to install persistent pH gradients of approximately 4 pH units under 500 nm light. When interfaced between two electrodes and exposed to asymmetric light irradiation, these solutions can be photoactivated precisely both in space and time, generating open circuit voltages as high as 240 mV that can last hours under steady-state irradiation - an outcome that is akin the peak performance of biological transmembrane proton pumps.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
LinkOut - more resources
Full Text Sources

