Prospective Application of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Disseminated Intravascular Coagulation
- PMID: 39569063
- PMCID: PMC11577934
- DOI: 10.2147/IJN.S467158
Prospective Application of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Disseminated Intravascular Coagulation
Abstract
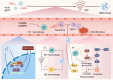
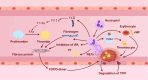
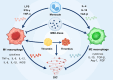
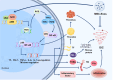
Disseminated intravascular coagulation (DIC) is an acquired disorder characterized by systemic activation of blood coagulation, which can arise from various causes. Owing to its abrupt onset, rapid progression, and high mortality rate, DIC presents a major clinical challenge. Anticoagulant drugs, such as heparin or low-molecular-weight heparin, are the current gold standard of treatment; however, these interventions pose considerable bleeding risks. Thus, safer and more effective therapeutic strategies are urgently required. Owing to their strong anti-inflammatory and tissue repair capabilities, mesenchymal stem cell-derived exosomes (MSC-Exos) have gained considerable attention as novel therapeutic options for numerous disorders, including DIC. Their stability in diverse pathological states highlights their potential as promising candidates for DIC therapy. This review presents the latest insights on the pathogenesis of DIC and anti-inflammatory and anticoagulant properties of MSC-Exos. We aimed to elucidate the potential mechanisms by which MSC-Exos influence DIC pathogenesis. We speculate that MSC-Exos offer a multifaceted approach to DIC treatment by attenuating neutrophil extracellular trap formation, modulating M1/M2 macrophage polarization, altering Nrf2/NF-κB signalling pathway to downregulate pro-inflammatory factors, and correcting imbalances in the coagulation-fibrinolysis system through anticoagulant routes. This suggests that MSC-Exos are a potential paradigm in DIC therapy, offering novel targets and treatment modalities for DIC management.
Keywords: anticoagulant therapy; disseminated intravascular coagulation; macrophage polarization; mesenchymal stem cell-derived exosomes; neutrophil extracellular traps.
© 2024 Wang et al.
Conflict of interest statement
The authors report no known conflicts of interest in this work.
Figures
Similar articles
-
Paeoniflorin alleviates lipopolysaccharide-induced disseminated intravascular coagulation by inhibiting inflammation and coagulation activation.Drug Dev Res. 2020 Jun;81(4):517-525. doi: 10.1002/ddr.21647. Epub 2020 Feb 17. Drug Dev Res. 2020. PMID: 32065451
-
Management of cancer-associated disseminated intravascular coagulation.Thromb Res. 2016 Apr;140 Suppl 1:S66-70. doi: 10.1016/S0049-3848(16)30101-3. Thromb Res. 2016. PMID: 27067981 Review.
-
Mesenchymal Stem Cell-Derived Exosomes Promote Recovery of The Facial Nerve Injury through Regulating Macrophage M1 and M2 Polarization by Targeting the P38 MAPK/NF-Κb Pathway.Aging Dis. 2024 Apr 1;15(2):851-868. doi: 10.14336/AD.2023.0719-1. Aging Dis. 2024. PMID: 37548941 Free PMC article.
-
Current drug treatment strategies for disseminated intravascular coagulation.Drugs. 1998 Jun;55(6):767-77. doi: 10.2165/00003495-199855060-00004. Drugs. 1998. PMID: 9617592 Review.
-
Therapeutic Strategies for Disseminated Intravascular Coagulation Associated with Aortic Aneurysm.Int J Mol Sci. 2022 Jan 24;23(3):1296. doi: 10.3390/ijms23031296. Int J Mol Sci. 2022. PMID: 35163216 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

