A sexually dimorphic signature of activity-dependent BDNF signaling on the intrinsic excitability of pyramidal neurons in the prefrontal cortex
- PMID: 39569070
- PMCID: PMC11576208
- DOI: 10.3389/fncel.2024.1496930
A sexually dimorphic signature of activity-dependent BDNF signaling on the intrinsic excitability of pyramidal neurons in the prefrontal cortex
Abstract
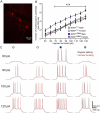
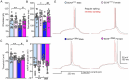
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders with strong genetic heterogeneity and more prevalent in males than females. We and others hypothesize that diminished activity-dependent neural signaling is a common molecular pathway dysregulated in ASD caused by diverse genetic mutations. Brain-derived neurotrophic factor (BDNF) is a key growth factor mediating activity-dependent neural signaling in the brain. A common single nucleotide polymorphism (SNP) in the pro-domain of the human BDNF gene that leads to a methionine (Met) substitution for valine (Val) at codon 66 (Val66Met) significantly decreases activity-dependent BDNF release without affecting basal BDNF secretion. By using mice with genetic knock-in of this human BDNF methionine (Met) allele, our previous studies have shown differential severity of autism-like social deficits in male and female BDNF+/Met mice. Pyramidal neurons are the principal neurons in the prefrontal cortex (PFC), a key brain region for social behaviors. Here, we investigated the impact of diminished activity-dependent BDNF signaling on the intrinsic excitability of pyramidal neurons in the PFC. Surprisingly, diminished activity-dependent BDNF signaling significantly increased the intrinsic excitability of pyramidal neurons in male mice, but not in female mice. Notably, significantly decreased thresholds of action potentials were observed in male BDNF+/Met mice, but not in female BDNF+/Met mice. Voltage-clamp recordings revealed that the sodium current densities were significantly increased in the pyramidal neurons of male BDNF+/Met mice, which were mediated by increased transcriptional level of Scn2a encoding sodium channel NaV 1.2. Medium after hyperpolarization (mAHP), another important parameter to determine intrinsic neuronal excitability, is strongly associated with neuronal firing frequency. Further, the amplitudes of mAHP were significantly decreased in male BDNF+/Met mice only, which were mediated by the downregulation of Kcnn2 encoding small conductance calcium-activated potassium channel 2 (SK2). This study reveals a sexually dimorphic signature of diminished activity-dependent BDNF signaling on the intrinsic neuronal excitability of pyramidal neurons in the PFC, which provides possible cellular and molecular mechanisms underpinning the sex differences in idiopathic ASD patients and human autism victims who carry BDNF Val66Met SNP.
Keywords: BDNF; Val66Met polymorphism; activity-dependent neural signaling; intrinsic neuronal excitability; sex.
Copyright © 2024 Ma, Zhang, McDaniel, Webb, Newton, Lee and Qin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Diminished activity-dependent BDNF signaling differentially causes autism-like behavioral deficits in male and female mice.Front Psychiatry. 2023 May 3;14:1182472. doi: 10.3389/fpsyt.2023.1182472. eCollection 2023. Front Psychiatry. 2023. PMID: 37205980 Free PMC article.
-
The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex.J Neurosci. 2012 Feb 15;32(7):2410-21. doi: 10.1523/JNEUROSCI.5205-11.2012. J Neurosci. 2012. PMID: 22396415 Free PMC article.
-
Dysregulated cortical excitability and tau phosphorylation in a β3 integrin mouse model of autism.Brain. 2025 Apr 10:awaf089. doi: 10.1093/brain/awaf089. Online ahead of print. Brain. 2025. PMID: 40209105
-
Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex.Biol Psychiatry. 2012 Jun 1;71(11):996-1005. doi: 10.1016/j.biopsych.2011.09.030. Epub 2011 Oct 29. Biol Psychiatry. 2012. PMID: 22036038 Free PMC article.
-
Alteration of the Centromedial Amygdala Glutamatergic Synapses by the BDNF Val66Met Polymorphism.Neuropsychopharmacology. 2015 Aug;40(9):2269-77. doi: 10.1038/npp.2015.76. Epub 2015 Mar 18. Neuropsychopharmacology. 2015. PMID: 25786582 Free PMC article.
Cited by
-
Chemogenetic Inhibition of Prefrontal Cortex Ameliorates Autism-Like Social Deficits and Absence-Like Seizures in a Gene-Trap Ash1l Haploinsufficiency Mouse Model.Genes (Basel). 2024 Dec 18;15(12):1619. doi: 10.3390/genes15121619. Genes (Basel). 2024. PMID: 39766886 Free PMC article.
References
-
- Bauer C. K., Schneeberger P. E., Kortüm F., Altmüller J., Santos-Simarro F., Baker L., et al. . (2019). Gain-of-function mutations in KCNN3 encoding the small-conductance ca(2+)-activated K(+) channel SK3 cause Zimmermann-Laband syndrome. Am. J. Hum. Genet. 104, 1139–1157. doi: 10.1016/j.ajhg.2019.04.012, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

