Revolutionizing environmental cleanup: the evolution of MOFs as catalysts for pollution remediation
- PMID: 39569125
- PMCID: PMC11578092
- DOI: 10.1039/d4ra05642f
Revolutionizing environmental cleanup: the evolution of MOFs as catalysts for pollution remediation
Abstract
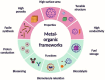
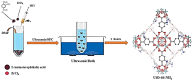
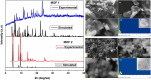
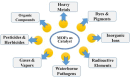
The global problem of ecological safety and public health necessitates, the development of new sustainable ideas for pollution remediation. In recent development, metal-organic frameworks (MOF) are the emerging technology with remarkable potential, which have been employed in environmental remediation. MOFs are networks that are created by the coordination of metals or polyanions with ligands and contain organic components that can be customized. The interesting features of MOFs are a large surface area, tuneable porosity, functional diversity, and high predictability of pollutant adsorption, catalysis, and degradation. It is a solid material that occupies a unique position in the war against environmental pollutants. One of the main benefits of MOFs is that they exhibit selective adsorption of a wide range of pollutants, including heavy metals, organics, greenhouse gases, water and soil. Only particles with the right combination of pore size and chemical composition will achieve this selectivity, derived from the high level of specificity. Besides, they possess high catalytic ability for the removal of pollutants by means of different methods such as photocatalysis, Fenton-like reactions, and oxidative degradation. By generating mobile active sites within the framework of MOFs, we can not only ensure high affinity for pollutants but also effective transformation of toxic chemicals into less harmful or even inert end products. However, the long-term stability of MOFs is becoming more important as eco-friendly parts are replaced with those that can be used repeatedly, and systems based on MOFs that can remove pollutants in more than one way are fabricated. MOFs can reduce waste production, energy consumption as compared to the other removal process. With its endless capacities, MOF technology brings a solution to the environmental cleansing problem, working as a flexible problem solver from one field to another. The investigation of MOF synthesis and principles will allow researchers to fully understand the potential of MOFs in environmental problem solving, making the world a better place for all of us.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures





















References
-
- Naidu R. Biswas B. Willett I. R. Cribb J. Singh B. K. Nathanail C. P. Coulon F. Semple K. T. Jones K. C. Barclay A. Environ. Int. 2021;156:106616. - PubMed
-
- Dhaka S. Kumar R. Deep A. Kurade M. B. Ji S.-W. Jeon B.-H. Coord. Chem. Rev. 2019;380:330–352.
-
- Felix Sahayaraj A. Joy Prabu H. Maniraj J. Kannan M. Bharathi M. Diwahar P. Salamon J. J. Inorg. Organomet. Polym. Mater. 2023;33:1757–1781.
-
- Jiao L. Jiang H.-L. Chin. J. Catal. 2023;45:1–5.
-
- Verma C. Rasheed T. Anwar M. T. Quraishi M. Microchem. J. 2023:108954.
Publication types
LinkOut - more resources
Full Text Sources

