Surface-modified CMOS biosensors
- PMID: 39569164
- PMCID: PMC11576298
- DOI: 10.3389/fbioe.2024.1441430
Surface-modified CMOS biosensors
Abstract
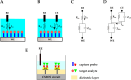
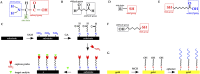
Biosensors translate biological events into electronic signals that quantify biological processes. They are increasingly used in in vitro diagnostics applications that leverage their ability to process small sample volumes. One recent trend has been to integrate biosensors with complementary metal-oxide-semiconductor (CMOS) chips to provide enhanced miniaturization, parallel sensing, and low power consumption at a low cost. CMOS-enabled biosensors are used in monitoring DNA hybridization, enzymatic reactions, and cell proliferation, to name a few applications. This paper explores the materials and processes used in emerging CMOS biosensors. We discuss subtractive and additive processes for creating electrodes for electrochemical sensing applications. We discuss functionalization techniques for creating bioelectronic interfaces that allow molecular events to be transduced into the electrical domain using a plurality of modalities that are readily provided by CMOS chips. Example modalities featured are optical sensing, electrochemical detection, electrical detection, magnetic sensing, and mechanical sensing.
Keywords: biosensor; complementary metal-oxide-semiconductor (CMOS); immobilization; lab-on-a-chip (LOC); post-CMOS process; transduction.
Copyright © 2024 Dehghandehnavi, Sajal and Dandin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

