NET-EN treatment leads to delayed HSV-2 infection, enhanced mucin and T cell functions in the female genital tract when compared to DMPA in a preclinical mouse model
- PMID: 39569191
- PMCID: PMC11576457
- DOI: 10.3389/fimmu.2024.1427842
NET-EN treatment leads to delayed HSV-2 infection, enhanced mucin and T cell functions in the female genital tract when compared to DMPA in a preclinical mouse model
Abstract
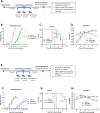
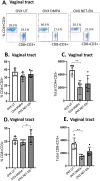
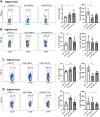
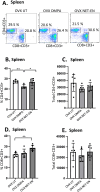
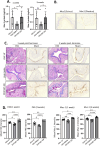
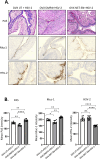
Depot-medroxyprogesterone acetate (DMPA) and Norethisterone Enanthate (NET-EN) are progestin-only injectable contraceptives widely used by women in sub-Sharan Africa, where incidence of HIV-1 and HSV-2 infection remains high. Studies indicate that DMPA usage can increase the risk of HSV-2 infection, but limited data indicate no increased risk with use of NET-EN. We therefore investigated the effects of NET-EN and DMPA on susceptibility to vaginal HSV-2 infection in ovariectomized (OVX) mice and effects on immune responses, particularly in the vaginal tract (VT). OVX mice, when treated with NET-EN and infected intravaginally, had delayed genital pathology, decreased viral shedding, and extended survival compared to DMPA- or untreated OVX mice. CD4+ T cells isolated from VT showed no significant change in frequency with either contraceptive. However, DMPA significantly decreased the total number of VT CD4+ and CD8+ T cells and the number of IFN-γ producing CD4 and CD8 T cells and increased the percentage of CD4 and CD8 T cells producing TNF-α compared to untreated mice. In contrast, NET-EN significantly enhanced percentages of CD8+ T cells compared to DMPA treated mice, and frequencies of IFN-γ+ CD4 and CD8 T cells in the VT compared to untreated mice. Comparative analysis of splenic lymphocytes indicated that DMPA treatment resulted in reduction of CD4+ T cell frequency, but enhanced TNF-α+ CD4 T cells compared to untreated mice. NET-EN enhanced the frequency of CD8 T cells, as well as IFN-γ+ and TNF-α+ CD4, and IFN-γ+ CD8 T cells in the spleen compared to untreated mice. Importantly, we found DMPA treatment that significantly reduced mucin production, whereas NET-EN enhanced expression of cell-associated mucin in VT. High levels of mucin in NET-EN mice were associated with lower levels of HSV-2 virus detected in the vaginal tract. This study provides the first evidence that NET-EN treatment can delay HSV-2 infection compared to DMPA.
Keywords: DMPA; IFN-γ; NET-EN; TNF-α; herpes simplex virus type 2; mucin; vaginal tract.
Copyright © 2024 Mian, Pa, Rahman, Gillgrass and Kaushic.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Quispe Calla NE, Vicetti Miguel RD, Torres AR, Trout W, Gabriel JM, Hatfield AM, et al. . Norethisterone enanthate increases mouse susceptibility to genital infection with herpes simplex virus type 2 and hiv type 1. Immunohorizons. (2020) 4:72–81. doi: 10.4049/immunohorizons.1900077 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

