Comparison of Hemodynamic Effects of Remimazolam and Midazolam During Anesthesia Induction in Patients Undergoing Cardiovascular Surgery: A Single-Center Retrospective and Exploratory Study
- PMID: 39569267
- PMCID: PMC11578151
- DOI: 10.7759/cureus.72032
Comparison of Hemodynamic Effects of Remimazolam and Midazolam During Anesthesia Induction in Patients Undergoing Cardiovascular Surgery: A Single-Center Retrospective and Exploratory Study
Abstract
Introduction: Patients undergoing cardiovascular surgery may experience hemodynamic instability during the induction of general anesthesia, and anesthetic agents with minimal hemodynamic effects should be administered. Midazolam, a classic benzodiazepine anesthetic, is known to have relatively weak circulatory depression during anesthesia induction compared to other sedatives. On the other hand, remimazolam, a newly approved short-acting benzodiazepine anesthetic, is expected to have fewer circulatory depressant effects. However, comparisons between remimazolam and midazolam regarding circulatory depression during anesthesia induction have not been adequately studied.
Objective: This study aims to compare the hemodynamic effects of remimazolam and midazolam during anesthesia induction in patients undergoing cardiovascular surgery.
Method: In this single-center retrospective and exploratory study, adults undergoing cardiovascular surgery under general anesthesia were divided into the remimazolam group (R group) and midazolam group (M group). Remimazolam 0.06 mg/kg (R group) or midazolam 0.03 mg/kg (M group) was administered during induction of general anesthesia. Both groups received remifentanil 1 μg/kg/min as analgesia. During anesthesia induction, additional sedatives (remimazolam or midazolam, respectively) were administered as needed to maintain the bispectral index (BIS) below 60. The primary endpoints were the following hemodynamic parameters: mean arterial pressure (MAP), heart rate (HR), cardiac index (CI), stroke volume index (SVI), systemic vascular resistance index (SVRI), and stroke volume variation (SVV). Measurements were taken before the induction of anesthesia, one and three minutes after rocuronium administration, and one, three, five, and 10 minutes after tracheal intubation. Secondary endpoints included the number of patients requiring vasopressors and vasopressor dosage, time to fall asleep, and BIS values. All values are expressed as the median (interquartile range). Continuous variables were compared using the Mann-Whitney U test. Statistically significant differences were set at p-values <0.05.
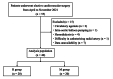
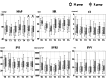
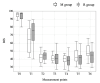
Results: Forty patients (20 in each group) were included in the final analysis. The doses of remimazolam and midazolam until sleep onset were 0.058 (0.053, 0.066) mg/kg in the R group and 0.035 (0.03, 0.045) mg/kg in the M group. The MAP at five minutes and 10 minutes after tracheal intubation was significantly higher in the R group than in the M group (p=0.031 and p=0.004, respectively). The HR, CI, SVI, SVRI, and SVV were not significantly different between the two groups at any of the measurement points. The number of patients requiring vasopressors and vasopressor dosage were not statistically significant between the two groups. The time to fall asleep was 124 seconds (90, 142) in the R group and 146 seconds (130, 167) in the M group, with a significant difference (p=0.01). The BIS values during anesthesia induction were not significantly different between the two groups.
Conclusion: Remimazolam had fewer hemodynamic effects than midazolam, even with relatively high doses and an earlier sleep onset. In terms of hemodynamic stability, remimazolam may be beneficial during anesthetic induction; however, further research is needed to confirm its efficacy.
Keywords: cardiac output monitoring; cardiovascular surgery; hemodynamic effects; midazolam; remimazolam.
Copyright © 2024, Shintani et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Institutional Review Board of Sasebo City General Hospital issued approval 2021-A027. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Midazolam: the first water-soluble benzodiazepine. Pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Kanto JH. Pharmacotherapy. 1985;5:138–155. - PubMed
-
- The effects of low-dose midazolam for induction of high-dose fentanyl anesthesia for coronary artery bypass graft. Kanaya N, Fujita S, Tsuchida H, Seki S, Namiki A. J Anesth. 1994;8:28–31. - PubMed
-
- Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! Masui K. J Anesth. 2020;34:479–482. - PubMed
-
- CNS 7056: a novel ultra-short-acting benzodiazepine. Kilpatrick GJ, McIntyre MS, Cox RF, et al. Anesthesiology. 2007;107:60–66. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
