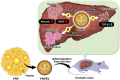
Platelet-rich plasma-derived extracellular vesicles improve liver cirrhosis in mice
- PMID: 39569343
- PMCID: PMC11576940
- DOI: 10.1016/j.reth.2024.10.010
Platelet-rich plasma-derived extracellular vesicles improve liver cirrhosis in mice
Abstract
Introduction: Cirrhosis remains a significant clinical challenge due to its poor prognosis and limited treatment options, creating a high unmet medical need for the development of novel therapies. In this study, we analyzed the effects of a novel approach to treat cirrhosis using platelet-rich plasma-derived extracellular vesicles (PRPEV) in mice.
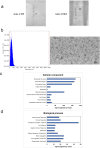
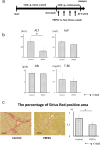
Methods: PRPEV were collected from platelet-rich plasma using ultrafiltration, and their proteomes were analyzed. The carbon tetrachloride (CCl4)-induced cirrhosis model of mice was used to evaluate the effect of PRPEV administration and compared with the control group (n = 8). In vitro and in vivo mechanistic analyses of PRPEV administration were confirmed using real time-PCR and immunostaining.
Results: Gene ontology analysis based on the proteome revealed that PRPEV contain many factors associated with EV and immune responses. In vitro, PRPEV polarize macrophages into an anti-inflammatory phenotype. Following PRPEV administration, there was a decrease in serum alanine aminotransferase levels and reduction in liver fibrosis, while mRNA levels of regenerative factors were upregulated and transforming growth factor β-1 was downregulated. Furthermore, the number of anti-inflammatory macrophages in the liver increased.
Conclusions: PRPEV may contribute to hepatocyte proliferation, anti-inflammation, and anti-fibrogenesis in the liver. This novel concept paves the way for cirrhosis treatment.
Keywords: Liver cirrhosis; Platelet; Platelet-rich plasma; Platelet-rich plasma-derived extracellular vesicles.
© 2024 The Author(s).
Conflict of interest statement
None.
Figures



References
-
- Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. - PubMed
-
- Zarrinpar A., Busuttil R.W. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol. 2013;10:434–440. - PubMed
-
- Lucey M.R., Furuya K.N., Foley D.P. Liver transplantation. N Engl J Med. 2023;389:1888–1900. - PubMed
-
- Underwood P.W., Cron D.C., Terjimanian M.N., Wang S.C., Englesbe M.J., Waits S.A. Sarcopenia and failure to rescue following liver transplantation. Clin Transplant. 2015;29:1076–1080. - PubMed
LinkOut - more resources
Full Text Sources

