The BNT162b2 mRNA vaccine demonstrates reduced age-associated TH1 support in vitro and in vivo
- PMID: 39569372
- PMCID: PMC11576392
- DOI: 10.1016/j.isci.2024.111055
The BNT162b2 mRNA vaccine demonstrates reduced age-associated TH1 support in vitro and in vivo
Abstract
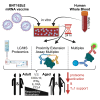
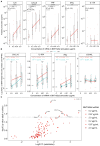
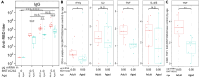
mRNA vaccines demonstrate impaired immunogenicity and durability in vulnerable older populations. We hypothesized that human in vitro modeling and proteomics could elucidate age-specific mRNA vaccine actions. BNT162b2-stimulation changed the plasma proteome of blood samples from young (18-50Y) and older adult (≥60Y) participants, assessed by mass spectrometry, proximity extension assay, and multiplex. Young adult up-regulation (e.g., PSMC6, CPN1) contrasted reduced induction in older adults (e.g., TPM4, APOF, APOC2, CPN1, PI16). 30-85% lower TH1-polarizing cytokines and chemokines were induced in elderly blood (e.g., IFNγ, CXCL10). Analytes lower in older adult samples included human in vivo mRNA immunogenicity biomarkers (e.g., IFNγ, CXCL10, CCL4, IL-1RA). BNT162b2 also demonstrated reduced CD4+ TH1 responses in aged vs. young adult mice. Our study demonstrates the utility of human in vitro platforms modeling age-specific mRNA vaccine immunogenicity, highlights impaired support of TH1 polarization in older adults, and provides a rationale for precision mRNA vaccine adjuvantation to induce greater immunogenicity.
Keywords: Geriatrics; Health sciences; Immunity; Immunology; Proteomics.
© 2024 The Author(s).
Conflict of interest statement
O.L. has served as a consultant to GlaxoSmithKline (GSK) and Hillevax. M.B.F. serves on the scientific advisory board of Aikido Pharma and has collaborative research agreements with Novavax, AstraZeneca, Regeneron, and Irazu Bio. B.B., E.N., T.R.O., D.S., S.H., O.L., and D.J.D. are named inventors on vaccine adjuvant patent(s). O.L., G.S.S., and D.J.D. are named inventors on patents related to human in vitro modeling of vaccine responses. O.L. and G.S.S. are recipients of a sponsored research agreement with GSK. D.J.D is on the scientific advisory board of EdJen BioTech and serves as a consultant with Merck Research Laboratories/Merck Sharp & Dohme Corp. (a subsidiary of Merck & Co., Inc.). O.L. and D.J.D. are co-founders of and advisors to Ovax, Inc. ACS and LRB are involved in HIV, COVID, and other vaccine clinical trials conducted in collaboration with the NIH, HIV Vaccine Trials Network (HVTN), COVID Vaccine Prevention Network (CoVPN), International AIDS Vaccine Initiative (IAVI), Crucell/Janssen, Moderna, and Sanofi. These commercial or financial relationships are unrelated to the current study. The participating Precision Vaccines Program (PVP) laboratories were supported in part, by U.S. National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Diseases (NIAID) awards, including Human Immunology Project Consortium award U19 AI118608, Adjuvant Discovery (HHSN272201400052C) and Development (HHSN272201800047C) Program Contracts to O.L.; Adjuvant Discovery Program contract (75N93019C00044) to O.L. and D.J.D as well as NIH grant (1R21AI137932-01A1) to D.J.D. O.L. is also funded by an award from the Coalition for Epidemic Preparedness Innovations (CEPI), via the International Network of Special Immunization Services (INSIS). The PVP is supported, in part, by the BCH Department of Pediatrics and philanthropy via the BCH Trust, including from the Barry Family and the Boston Investment Council. A.K.C. was supported by the Friedman Award for Scholars in Health, the University of British Columbia, and Mitacs Accelerate Canada.
Figures



References
-
- Arregoces-Castillo L., Fernandez-Nino J., Rojas-Botero M., Palacios-Clavijo A., Galvis-Pedraza M., Rincon-Medrano L., Pinto-Alvarez M., Ruiz-Gomez F., Trejo-Valdivia B. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022;3:e242–e252. doi: 10.1016/S2666-7568(22)00035-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

