Inhibiting AMPA receptor signaling in oligodendrocytes rescues synapse loss in a model of autoimmune demyelination
- PMID: 39569383
- PMCID: PMC11577175
- DOI: 10.1016/j.isci.2024.111226
Inhibiting AMPA receptor signaling in oligodendrocytes rescues synapse loss in a model of autoimmune demyelination
Abstract
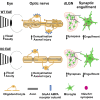
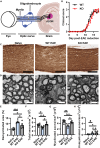
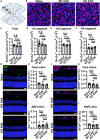
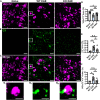
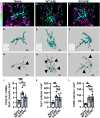
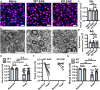
Multiple sclerosis (MS) is initially characterized by myelin and axonal damage in central nervous system white matter lesions, but their causal role in synapse loss remains undefined. Gray matter atrophy is also present early in MS, making it unclear if synaptic alterations are driven by white matter demyelinating lesions or primary gray matter damage. Furthermore, whether axonal pathology occurs secondary to or independent of demyelination to drive synaptic changes is not clear. Here, we address whether reducing demyelination by selectively manipulating glutamatergic signaling in mature oligodendrocytes (OLs) preserves synapses in experimental autoimmune encephalomyelitis (EAE), a preclinical model of demyelinating disease. We demonstrate that inducible reduction of the GluA4 AMPA-type glutamate receptor subunit selectively in mature (OLs) reduces demyelination and axonal injury, preserves synapses, and improves visual function during EAE. These data link demyelination to the pathophysiology of synaptic loss with therapeutic implications for both motor and cognitive disability in MS.
Keywords: Cell biology; Immunology; Neuroscience.
© 2024 The Author(s).
Conflict of interest statement
T.M.D. serves on the board of directors for Coeptis Therapeutics.
Figures
References
-
- Werneburg S., Jung J., Kunjamma R.B., Ha S.K., Luciano N.J., Willis C.M., Gao G., Biscola N.P., Havton L.A., Crocker S.J., et al. Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity. 2020;52:167–182.e7. doi: 10.1016/j.immuni.2019.12.004. - DOI - PMC - PubMed
-
- Mey G.M., Evonuk K.S., Chappell M.K., Wolfe L.M., Singh R., Batoki J.C., Yu M., Peachey N.S., Anand-Apte B., Bermel R., et al. Visual imaging as a predictor of neurodegeneration in experimental autoimmune demyelination and multiple sclerosis. Acta Neuropathol. Commun. 2022;10:87. doi: 10.1186/s40478-022-01391-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

