Effectiveness and Safety of Glecaprevir/Pibrentasvir in Italian Children and Adolescents With Chronic Hepatitis C: A Real-Word, Multicenter Study
- PMID: 39569493
- PMCID: PMC11897846
- DOI: 10.1111/liv.16180
Effectiveness and Safety of Glecaprevir/Pibrentasvir in Italian Children and Adolescents With Chronic Hepatitis C: A Real-Word, Multicenter Study
Abstract
Background & aims: Glecaprevir/Pibrentasvir (GLE/PIB) has been approved by the European Medicine Agency (EMA) and by the US Food and Drug Administration (US-FDA) for the treatment of children and adolescents from 3 years of age with chronic hepatitis C virus (CHC) infection. The aim of this study was to confirm the real-world effectiveness and safety of GLE/PIB in children and adolescents (3 to < 18 years old) with CHC.
Methods: This prospective, multicentre study involved 11 Italian centres. Children and adolescents (from 3 to < 18 years of age) received a weight-based dose (up to 300/120 mg) of GLE/PIB once daily for 8 weeks. The effectiveness endpoint was sustained virological response 12 weeks after the end of treatment (SVR12). Safety was assessed by adverse events (AE) and clinical/laboratory data.
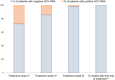
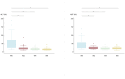
Results: Sixty-one patients (median age 12 years, interquartile range 5) were enrolled and treated between June 2020 and October 2023. Genotype distribution was as follows: 24/61 genotype 1 (39.4%), 13/61 genotype 2 (21.3%), 18/61 genotype 3 (29.5%) and 6/61 genotype 4 (9.8%). Sixty (98.4%) patients completed treatment and follow-up. SVR12 was obtained by 60/61 patients (98.4%). One patient died because of an oncological illness while on treatment. AE occurred in 13.1% of the patients, were mild and no patients prematurely stopped treatment.
Conclusions: This study confirmed the real-life effectiveness and safety of the 8-week therapy with GLE/PIB for treatment of CHC in children and adolescents.
Keywords: child; direct‐acting antivirals; hepatitis C virus; pan‐genotypic regimen; sustained virological response.
© 2024 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- 2023 Update of The World Health Organisation , WHO Global Hepatitis Report, 2017 (Geneva: World Health Organization, 2017), https://www.who.int/news‐room/fact‐sheets/detail/hepatitis‐c.
-
- Indolfi G., Easterbrook P., Dusheiko G., et al., “Hepatitis C Virus Infection in Children and Adolescents,” Lancet Gastroenterology & Hepatology 4 (2019): 477–487. - PubMed
-
- Indolfi G., Mangone G., Bartolini E., Moriondo M., Azzari C., and Resti M., “Hepatitis C Viraemia After Apparent Spontaneous Clearance in a Vertically Infected Child,” Lancet 387 (2016): 1967–1968. - PubMed
-
- European Paediatric Hepatitis C Virus Network , “Three Broad Modalities in the Natural History of Vertically Acquired Hepatitis C Virus Infection,” Clinical Infectious Diseases 41 (2005): 45–51. - PubMed
-
- Resti M., Jara P., Hierro L., et al., “Clinical Features and Progression of Perinatally Acquired Hepatitis C Virus Infection,” Journal of Medical Virology 70 (2003): 373–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

