A Latent Cardiomyocyte Regeneration Potential in Human Heart Disease
- PMID: 39569515
- PMCID: PMC11748904
- DOI: 10.1161/CIRCULATIONAHA.123.067156
A Latent Cardiomyocyte Regeneration Potential in Human Heart Disease
Abstract
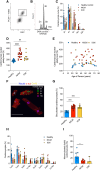
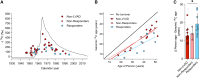
Background: Cardiomyocytes in the adult human heart show a regenerative capacity, with an annual renewal rate of ≈0.5%. Whether this regenerative capacity of human cardiomyocytes is employed in heart failure has been controversial.
Methods: We determined cardiomyocyte renewal in 52 patients with advanced heart failure, 28 of whom received left ventricular assist device support. We measured the concentration of nuclear bomb test-derived 14C in cardiomyocyte genomic DNA and performed mathematical modeling to establish cardiomyocyte renewal in heart failure with and without LVAD unloading.
Results: We show that cardiomyocyte generation is minimal in end-stage heart failure patients at rates 18 to 50× lower compared with the healthy heart. However, patients receiving left ventricle support device therapy, who showed significant functional and structural cardiac improvement, had a >6-fold increase in cardiomyocyte renewal relative to the healthy heart.
Conclusions: Our findings reveal a substantial cardiomyocyte regeneration potential in human heart disease, which could be exploited therapeutically.
Keywords: heart failure; heart-assist device ◼ polyploidy ◼ regeneration; myocytes, cardiac.
Conflict of interest statement
None.
Figures



Update of
-
A latent cardiomyocyte regeneration potential in human heart disease.bioRxiv [Preprint]. 2023 Sep 17:2023.09.14.557681. doi: 10.1101/2023.09.14.557681. bioRxiv. 2023. Update in: Circulation. 2025 Jan 21;151(3):245-256. doi: 10.1161/CIRCULATIONAHA.123.067156. PMID: 37745322 Free PMC article. Updated. Preprint.
References
-
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, et al. . Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026 - PubMed
-
- Chien KR, Frisen J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol. 2019;37:232–237. doi: 10.1038/s41587-019-0042-1 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

