Sustainable production of aromatic chemicals from lignin using enzymes and engineered microbes
- PMID: 39569570
- PMCID: PMC11580001
- DOI: 10.1039/d4cc05064a
Sustainable production of aromatic chemicals from lignin using enzymes and engineered microbes
Abstract
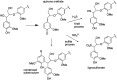
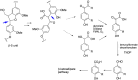
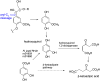
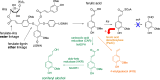
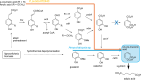
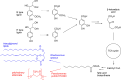
Lignin is an aromatic biopolymer found in plant cell walls and is the most abundant source of renewable aromatic carbon in the biosphere. Hence there is considerable interest in the conversion of lignin, either derived from agricultural waste or produced as a byproduct of pulp/paper manufacture, into high-value chemicals. Although lignin is rather inert, due to the presence of ether C-O and C-C linkages, several microbes are able to degrade lignin. This review will introduce these microbes and the enzymes that they use to degrade lignin and will describe recent studies on metabolic engineering that can generate high-value chemicals from lignin bioconversion. Catabolic pathways for degradation of lignin fragments will be introduced, and case studies where these pathways have been engineered by gene knockout/insertion to generate bioproducts that are of interest as monomers for bioplastic synthesis or aroma chemicals will be described. Life cycle analysis of lignin bioconversion processes is discussed.
Conflict of interest statement
There are no conflicts to declare.
Figures








Similar articles
-
Enzymatic conversion of lignin into renewable chemicals.Curr Opin Chem Biol. 2015 Dec;29:10-7. doi: 10.1016/j.cbpa.2015.06.009. Epub 2015 Jun 26. Curr Opin Chem Biol. 2015. PMID: 26121945 Review.
-
The chemical logic of enzymatic lignin degradation.Chem Commun (Camb). 2024 Jan 18;60(7):804-814. doi: 10.1039/d3cc05298b. Chem Commun (Camb). 2024. PMID: 38165282 Free PMC article. Review.
-
Construction and Optimization of a Heterologous Pathway for Protocatechuate Catabolism in Escherichia coli Enables Bioconversion of Model Aromatic Compounds.Appl Environ Microbiol. 2017 Aug 31;83(18):e01313-17. doi: 10.1128/AEM.01313-17. Print 2017 Sep 15. Appl Environ Microbiol. 2017. PMID: 28733280 Free PMC article.
-
Recent advances in enzymes active on lignin-derived aromatic compounds.Trends Biochem Sci. 2025 Apr;50(4):322-331. doi: 10.1016/j.tibs.2025.01.005. Epub 2025 Feb 13. Trends Biochem Sci. 2025. PMID: 39952881 Review.
-
Catabolism of β-5 linked aromatics by Novosphingobium aromaticivorans.mBio. 2024 Aug 14;15(8):e0171824. doi: 10.1128/mbio.01718-24. Epub 2024 Jul 16. mBio. 2024. PMID: 39012147 Free PMC article.
References
-
- “Carbon for Chemicals: how can biomass contribute to the defossilisation of the chemicals sector?” Report by SuperGen Bioenergy and Biomass Biorefinery Network, 2024, ISBN: 978-1-85449-820-5
-
- Fiorentino G. Ripa M. Ulgiati S. Biofuels, Bioprod. Biorefin. 2017;11:195–214. doi: 10.1002/bbb.1729. - DOI
-
- Rishikesh M. S. Harish S. Prasanth S. M. Prakash D. G. Biomass Convers. Biorefin. 2023;13:5533–5556. doi: 10.1007/s13399-021-01637-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

