MXene-Graphene Oxide Heterostructured Films for Enhanced Metasurface Plasmonic Biosensing in Continuous Glucose Monitoring
- PMID: 39569760
- PMCID: PMC11775529
- DOI: 10.1002/advs.202410376
MXene-Graphene Oxide Heterostructured Films for Enhanced Metasurface Plasmonic Biosensing in Continuous Glucose Monitoring
Abstract
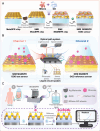
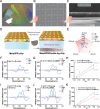
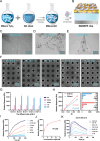
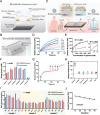
Non-invasive biosensors have attracted attention for their potential to obtain continuous, real-time physiological information through measurements of biochemical markers, such as one of the most important-glucose, in biological fluids. Although some optical sensing materials are used in non-invasive devices for continuous glucose monitoring (CGM), surface or localized plasmon sensing material are seldom applied in CGM owing to modest sensitivity and bulk sensing apparatus. Herein, a metasurface (MGMSPR) biosensor based on the metasurface plasmon resonance chip modified with heterostructured Ti3C2 MXene-Graphene oxide (MG) is reported, which potentially enables ultra-sensitive glucose detection. The sensor consists of a dual-channel microfluidic device integrated with silver mirror enhanced MGMSPR chips. Not only does it promote the entry of glucose oxidase (GOD) into the internal pores and enhance the stable fixation of GOD in the membrane, but also the integration of MG material provides a high specific surface area and unique electronic properties, thereby significantly enhancing the sensitivity of the MGMSPR sensor. The detection limit of MGMSPR biosensor is 106.8 µM. This pioneering approach opens new avenues for monitoring physiological parameters and process analytical technology on an optical platform, providing continuous health monitoring and production process control through optical sensors.
Keywords: MXene; glucose monitoring; heterostructured material; metasurface plasmonic sensor; microfluidics.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
High-Electron-Mobility MXene-Enhanced Metasurface Biosensors Integrated with Microfluidics for Real-Time Multifunctional Monitoring.ACS Nano. 2025 Apr 1;19(12):12007-12020. doi: 10.1021/acsnano.4c17289. Epub 2025 Mar 24. ACS Nano. 2025. PMID: 40126942
-
Wearable MXene-enhanced organic Bio-FET paper patch for glucose detection in sweat with pH and temperature calibration.Sci Rep. 2025 May 9;15(1):16219. doi: 10.1038/s41598-025-00533-1. Sci Rep. 2025. PMID: 40346101 Free PMC article.
-
Ultra-sensitive surface plasmon resonance sensor integrating MXene (Ti3C2TX) and graphene for advanced carcinoembryonic antigen detection.Sci Rep. 2025 Apr 19;15(1):13571. doi: 10.1038/s41598-025-97853-z. Sci Rep. 2025. PMID: 40253475 Free PMC article.
-
2D Material-Based Optical Biosensor: Status and Prospect.Adv Sci (Weinh). 2022 Feb;9(4):e2102924. doi: 10.1002/advs.202102924. Epub 2021 Dec 13. Adv Sci (Weinh). 2022. PMID: 34898053 Free PMC article. Review.
-
2D material-based surface plasmon resonance biosensors for applications in different domains: an insight.Mikrochim Acta. 2024 Jun 6;191(7):373. doi: 10.1007/s00604-024-06442-w. Mikrochim Acta. 2024. PMID: 38842697 Review.
References
-
- a) Liu Y., Pharr M., Salvatore G. A., ACS Nano 2017, 11, 9614; - PubMed
- b) Amjadi M., Kyung K.‐U., Park I., Sitti M., Adv. Funct. Mater. 2016, 26, 1678;
- c) Bariya M., Nyein H. Y. Y., Javey A., Nat. Electron. 2018, 1, 160;
- d) Kim J., Campbell A. S., de Ávila B. E.‐F., Wang J., Nat. Biotechnol. 2019, 37, 389; - PMC - PubMed
- e) Xue F., Zhao S., Tian H., Qin H., Li X., Jian Z., Du J., Li Y., Wang Y., Lin L., Liu C., Shang Y., He L., Xing M., Zeng W., Nat. Commun. 2024, 15, 864. - PMC - PubMed
-
- a) Gao F., Liu C., Zhang L., Liu T., Wang Z., Song Z., Cai H., Fang Z., Chen J., Wang J., Han M., Wang J., Lin K., Wang R., Li M., Mei Q., Ma X., Liang S., Gou G., Xue N., Microsyst. Nanoeng. 2023, 9, 10.1038/s41378-022-00443-6. - DOI - PMC - PubMed
- b) Emaminejad S., Gao W., Wu E., Davies Z. A., Yin Yin Nyein H., Challa S., Ryan S. P., Fahad H. M., Chen K., Shahpar Z., Talebi S., Milla C., Javey A., Davis R. W., Proc. Natl. Acad. Sci. USA 2017, 114, 4625. - PMC - PubMed
-
- García‐Carmona L., Martín A., Sempionatto J. R., Moreto J. R., González M. C., Wang J., Escarpa A., Anal. Chem. 2019, 91, 13883. - PubMed
-
- Chen S., Qiao Z., Niu Y., Yeo J. C., Liu Y., Qi J., Fan S., Liu X., Lee J. Y., Lim C. T., Nat. Rev. Bioeng. 2023, 1, 950.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
