Exploring the Antifibrotic Mechanisms of Ghrelin: Modulating TGF-β Signalling in Organ Fibrosis
- PMID: 39569809
- PMCID: PMC11879379
- DOI: 10.1017/erm.2024.38
Exploring the Antifibrotic Mechanisms of Ghrelin: Modulating TGF-β Signalling in Organ Fibrosis
Abstract
Background: Fibrosis is a pathological condition that affects various organs by increasing fibrous connective tissue while reducing parenchymal cells. This imbalance can lead to compromised organ function and potential failure, posing significant health risks. The condition's complexity necessitates the exploration of effective treatments to mitigate its progression and adverse outcomes.
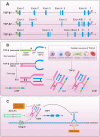
Aims: This study aims to investigate the role of ghrelin, a peptide hormone known for its anti-inflammatory and anti-fibrotic properties, in modulating fibrosis across different organs. By binding to the growth hormone secretagogue receptor type 1a (GHSR-1a), ghrelin has shown potential in attenuating the fibrotic process, particularly through its interaction with the TGF-β signalling pathway.
Methods: An extensive review of clinical and animal model studies focusing on liver, kidney, lung, and myocardial fibrosis was conducted. The primary focus was on examining how ghrelin influences the TGF-β signalling pathway, with an emphasis on the regulation of TGF-β expression and the suppression of Smad signalling molecules. The methodology involved analysing data from various studies to understand ghrelin's molecular mechanisms in combating fibrosis.
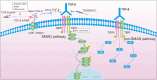
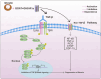
Results: The findings from the reviewed studies indicate that ghrelin exerts significant anti-fibrotic effects across multiple organ systems. Specifically, ghrelin was found to downregulate TGF-β expression and suppress Smad signalling molecules, leading to a marked reduction in fibrous tissue accumulation and preservation of organ function. In liver fibrosis models, ghrelin reduced TGF-β1 levels and Smad3 phosphorylation, while in kidney and cardiac fibrosis, similar protective effects were observed. The data also suggest that ghrelin's effects are mediated through both canonical and non-canonical TGF-β pathways.
Conclusions: Ghrelin presents a promising therapeutic agent in the management of fibrosis due to its potent anti-inflammatory and anti-fibrotic actions. Its ability to modulate the TGF-β signalling pathway underscores a vital molecular mechanism through which ghrelin can mitigate fibrotic progression in various organs. Future research should focus on further elucidating ghrelin's molecular interactions and potential clinical applications in fibrosis treatment, offering new avenues for developing effective anti-fibrotic therapies.
Keywords: TGF-β; antifibrotic mechanisms; fibrosis; ghrelin; organ.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Ghrelin ameliorates transformation of hepatic ischemia-reperfusion injury to liver fibrosis by blocking Smad and ERK signalling pathways, and promoting anti-inflammation and anti-oxidation effects.Transpl Immunol. 2022 Aug;73:101597. doi: 10.1016/j.trim.2022.101597. Epub 2022 Apr 3. Transpl Immunol. 2022. PMID: 35385777
-
Ghrelin Attenuates Liver Fibrosis through Regulation of TGF-β1 Expression and Autophagy.Int J Mol Sci. 2015 Sep 10;16(9):21911-30. doi: 10.3390/ijms160921911. Int J Mol Sci. 2015. PMID: 26378522 Free PMC article.
-
Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment.Biomed Pharmacother. 2018 May;101:670-681. doi: 10.1016/j.biopha.2018.02.090. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29518614 Review.
-
Transforming growth factor-beta and Smad signalling in kidney diseases.Nephrology (Carlton). 2005 Feb;10(1):48-56. doi: 10.1111/j.1440-1797.2005.00334.x. Nephrology (Carlton). 2005. PMID: 15705182 Review.
-
Harnessing nature's arsenal: Targeting the TGF-β/Smad Cascade with novel natural anti-fibrotic agents.Fitoterapia. 2025 Mar;181:106372. doi: 10.1016/j.fitote.2024.106372. Epub 2025 Jan 6. Fitoterapia. 2025. PMID: 39778722 Review.
References
-
- Xu M, et al. (2024) Mechanisms of fibrosis in iatrogenic laryngotracheal stenosis: New discoveries and novel targets. Biomedicine & Pharmacotherapy 170, 115995. - PubMed
-
- Ricard-Blum S and Miele AE (2020) Omic approaches to decipher the molecular mechanisms of fibrosis, and design new anti-fibrotic strategies. Seminars in Cell & Developmental Biology 101, 161–169. - PubMed
-
- Kojima M, et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402(6762), 656–660. - PubMed
-
- Uchida Y and Izumizaki M (2023) Modulation of acyl and des-acyl ghrelin on autonomic and behavioral thermoregulation in various ambient temperatures. Journal of Thermal Biology 113, 103543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

