Bystanders or active players: the role of extra centrosomes as signaling hubs
- PMID: 39570514
- PMCID: PMC11582193
- DOI: 10.1007/s10555-024-10224-4
Bystanders or active players: the role of extra centrosomes as signaling hubs
Abstract
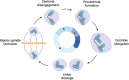
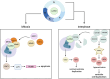
Centrosomes serve as microtubule-organizing organelles that function in spindle pole organization, cell cycle progression, and cilia formation. A non-canonical role of centrosomes that has gained traction in recent years is the ability to act as signal transduction centers. Centrosome amplification, which includes numerical and structural aberrations of centrosomes, is a candidate hallmark of cancer. The function of centrosomes as signaling centers in cancer cells with centrosome amplification is poorly understood. Establishing a model of how cancer cells utilize centrosomes as signaling platforms will help elucidate the role of extra centrosomes in cancer cell survival and tumorigenesis. Centrosomes act in a diverse array of cellular processes, including cell migration, cell cycle progression, and proteasomal degradation. Given that cancer cells with amplified centrosomes exhibit an increased number and larger area of these signaling platforms, extra centrosomes may be acting to promote tumor development by enhancing signaling kinetics in pathways that are essential for the formation and growth of cancer. In this review, we identify the processes centrosomes are involved in as signal transduction platforms and highlight ways in which cancer cells with centrosome amplification may be taking advantage of these mechanisms.
Keywords: Cancer; Cell cycle; Centrosome amplification; Centrosomes; Signaling.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: K.J.P. discloses that he is a consultant to Cue Biopharma, Inc., an equity holder in PEEL therapeutics, and a founder and equity holder in Keystone Biopharma, Inc, and Kreftect, Inc. S.R.A. discloses that she is an equity holder in Keystone Biopharma, Inc.
Figures

Similar articles
-
Dividing with Extra Centrosomes: A Double Edged Sword for Cancer Cells.Adv Exp Med Biol. 2017;1002:47-67. doi: 10.1007/978-3-319-57127-0_3. Adv Exp Med Biol. 2017. PMID: 28600782 Review.
-
Heading off with the herd: how cancer cells might maneuver supernumerary centrosomes for directional migration.Cancer Metastasis Rev. 2013 Jun;32(1-2):269-87. doi: 10.1007/s10555-012-9413-5. Cancer Metastasis Rev. 2013. PMID: 23114845 Free PMC article. Review.
-
Amplified centrosomes-more than just a threat.EMBO Rep. 2024 Oct;25(10):4153-4167. doi: 10.1038/s44319-024-00260-0. Epub 2024 Sep 16. EMBO Rep. 2024. PMID: 39285247 Free PMC article. Review.
-
The interplay between centrosomes and the Hippo tumor suppressor pathway.Chromosome Res. 2016 Jan;24(1):93-104. doi: 10.1007/s10577-015-9502-8. Chromosome Res. 2016. PMID: 26582635 Free PMC article. Review.
-
Using Cell Culture Models of Centrosome Amplification to Study Centrosome Clustering in Cancer.Methods Mol Biol. 2016;1413:367-92. doi: 10.1007/978-1-4939-3542-0_23. Methods Mol Biol. 2016. PMID: 27193861
References
-
- Nigg, E. A., Schäfer, G., Hilz, H., & Eppenberger, H. M. (1985). Cyclic-AMP-dependent protein kinase type II is associated with the Golgi complex and with centrosomes. Cell,41(3), 1039–1051. 10.1016/S0092-8674(85)80084-2 - PubMed
-
- Nigg, E. A., Schafer, G., & Eppenberger, H. M. (1986). Heterogeneous distribution of the cAMP receptor protein RII in the nervous system: Evidence for its intracellular accumulation on microtubules, microtubule-organizing centers, and in the area of the Golgi complex. The Journal of Cell Biology,103(1), 189–203. - PMC - PubMed
-
- Černohorská, M., Sulimenko, V., Hájková, Z., Sulimenko, T., Sládková, V., Vinopal, S., … & Dráber, P. (2016). GIT1/βPIX signaling proteins and PAK1 kinase regulate microtubule nucleation. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1863(6, Part A), 1282–1297. 10.1016/j.bbamcr.2016.03.016 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

