First-Line Systemic Treatment for Initially Unresectable Colorectal Liver Metastases: Post Hoc Analysis of the CAIRO5 Randomized Clinical Trial
- PMID: 39570583
- PMCID: PMC11583021
- DOI: 10.1001/jamaoncol.2024.5174
First-Line Systemic Treatment for Initially Unresectable Colorectal Liver Metastases: Post Hoc Analysis of the CAIRO5 Randomized Clinical Trial
Erratum in
-
Errors in Figures 2 and 3.JAMA Oncol. 2025 Jan 1;11(1):80. doi: 10.1001/jamaoncol.2024.6650. JAMA Oncol. 2025. PMID: 39821218 Free PMC article. No abstract available.
Abstract
Importance: In patients with colorectal cancer and unresectable liver-only metastases (CRLM), treatment with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) plus irinotecan (FOLFOXIRI) and bevacizumab vs FOLFOX/folinic acid, fluorouracil, and irinotecan (FOLFIRI) plus bevacizumab increased progression-free survival, response, and R0/R1 resection/ablation rates, as well as toxic effects in RAS/BRAFV600E-variant and/or right-sided tumors. FOLFOX/FOLFIRI-panitumumab vs FOLFOX/FOLFIRI-bevacizumab increased response at the cost of more toxic effects in RAS/BRAFV600E wild-type, left-sided tumors.
Objective: To present long-term outcomes of treatment with FOLFOXIRI plus bevacizumab vs FOLFOX/FOLFIRI plus bevacizumab and FOLFOX/FOLFIRI plus panitumumab vs FOLFOX/FOLFIRI + bevacizumab.
Design, setting, and participants: The randomized phase 3 CAIRO5 trial included patients with initially unresectable CRLM in 46 Dutch centers and 1 Belgian center between November 2014 and January 2022. A liver expert panel repeatedly evaluated resectability.
Intervention: Patients with RAS/BRAFV600E-variant and/or right-sided tumors randomly received FOLFOX/FOLFIRI-bevacizumab (group 1) or FOLFOXIRI-bevacizumab (group 2), and those with RAS/BRAFV600E wild-type, left-sided tumors received FOLFOX/FOLFIRI-bevacizumab (group 3) or FOLFOX/FOLFIRI-panitumumab (group 4). Adjuvant chemotherapy (ACT) after complete local treatment was recommended but not standard.
Main outcomes and measures: Overall survival (OS) was analyzed as a secondary outcome. Other outcomes were post hoc analyses.
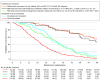
Results: A total of 530 patients (327 male [62%] and 203 female individuals [38%]; median age, 62 [IQR, 54-69] years) were randomized: 148 in group 1, 146 in group 2, 118 in group 3, and 118 in group 4. The median OS in group 1 was 23.6 (95% CI, 20.1-27.5) vs 24.1 (95% CI, 21.0-30.9) months in group 2 (hazard ratio [HR], 0.90; 95% CI, 0.70-1.17; P = .44), and 39.9 (95% CI, 30.7-44.6) in group 3 vs 38.3 (95% CI, 35.3-51.3) months in group 4 (HR, 0.95; 95% CI, 0.68-1.32; P = .75). OS was longest after complete local treatment without early (≤6 months) recurrence (64.3 months; 95% CI, 57.6 to not reached) and salvage local treatment options after early recurrence (58.9; 95% CI, 47.3 to not reached), followed by patients without salvage local treatment after early recurrence (30.5; 95% CI, 24.4-33.4) and with incomplete local treatment (28.7; 95% CI, 25.9-38.3), and worst in patients with continued unresectability (18.3; 95% CI, 15.7-20.0). After confounder adjustment, ACT was associated with longer OS (HR, 0.66; 95% CI, 0.44-0.98) and relapse-free survival (HR, 0.65; 95% CI, 0.48-0.88) and less early recurrence without salvage local treatment (odds ratio, 0.46; 95% CI, 0.25-0.85).
Conclusions and relevance: These results support using FOLFOX/FOLFIRI-bevacizumab for patients with initially unresectable CRLM irrespective of RAS/BRAFV600E status and tumor sidedness. Patients with complete local liver treatment with salvage local treatment in case of early recurrence had the longest OS. ACT might be considered for these patients.
Trial registration: ClinicalTrials.gov NCT02162563.
Conflict of interest statement
Figures

Comment in
-
The surgical perspective on CAIRO5 and beyond: all patients with colorectal liver metastases should be evaluated by a liver surgeon, but what defines resectability?J Gastrointest Oncol. 2025 Aug 30;16(4):1768-1772. doi: 10.21037/jgo-2025-291. Epub 2025 Aug 7. J Gastrointest Oncol. 2025. PMID: 40950334 Free PMC article. No abstract available.
-
Rethinking surrogate endpoints in metastatic colorectal cancer: counting chickens only when they hatch.J Gastrointest Oncol. 2025 Aug 30;16(4):1763-1767. doi: 10.21037/jgo-2025-386. Epub 2025 Aug 25. J Gastrointest Oncol. 2025. PMID: 40950350 Free PMC article. No abstract available.
Comment on
-
FOLFOX/FOLFIRI-Bevacizumab for Unresectable Colorectal Liver Metastases.JAMA Oncol. 2025 Jan 1;11(1):13-15. doi: 10.1001/jamaoncol.2024.5073. JAMA Oncol. 2025. PMID: 39570610 No abstract available.
References
-
- Bond MJG, Bolhuis K, Loosveld OJL, et al. ; Dutch Colorectal Cancer Study Group . First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): an open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023;24(7):757-771. doi: 10.1016/S1470-2045(23)00219-X - DOI - PubMed
-
- Lam VWT, Spiro C, Laurence JM, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19(4):1292-1301. doi: 10.1245/s10434-011-2061-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

