Estimated Effectiveness of Influenza Vaccines in Preventing Secondary Infections in Households
- PMID: 39570586
- PMCID: PMC11582933
- DOI: 10.1001/jamanetworkopen.2024.46814
Estimated Effectiveness of Influenza Vaccines in Preventing Secondary Infections in Households
Erratum in
-
Errors in Abstract Results.JAMA Netw Open. 2025 Jan 2;8(1):e2459710. doi: 10.1001/jamanetworkopen.2024.59710. JAMA Netw Open. 2025. PMID: 39813039 Free PMC article. No abstract available.
Abstract
Importance: Influenza vaccine effectiveness (VE) is commonly assessed against prevention of illness that requires medical attention. Few studies have evaluated VE against secondary influenza infections.
Objective: To determine the estimated effectiveness of influenza vaccines in preventing secondary infections after influenza was introduced into households.
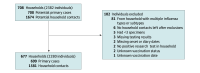
Design, settings, and participants: During 3 consecutive influenza seasons (2017-2020), primary cases (the first household members with laboratory-confirmed influenza) and their household contacts in Tennessee and Wisconsin were enrolled into a prospective case-ascertained household transmission cohort study. Participants collected daily symptom diaries and nasal swabs for up to 7 days. Data were analyzed from September 2022 to February 2024.
Exposures: Vaccination history, self-reported and verified through review of medical and registry records.
Main outcomes and measures: Specimens were tested using reverse transcription-polymerase chain reaction to determine influenza infection. Longitudinal chain binomial models were used to estimate secondary infection risk and the effectiveness of influenza vaccines in preventing infection among household contacts overall and by virus type and subtype and/or lineage.
Results: The analysis included 699 primary cases and 1581 household contacts. The median (IQR) age of the primary cases was 13 (7-38) years, 381 (54.5%) were female, 60 (8.6%) were Hispanic, 46 (6.6%) were non-Hispanic Black, 553 (79.1%) were Non-Hispanic White, and 343 (49.1%) were vaccinated. Among household contacts, the median age was 31 (10-41) years, 833 (52.7%) were female, 116 (7.3%) were Hispanic, 78 (4.9%) were non-Hispanic Black, 1283 (81.2%) were non-Hispanic White, 792 (50.1%) were vaccinated, and 356 (22.5%) had laboratory-confirmed influenza during follow-up. The overall secondary infection risk of influenza among household contacts was 18.8% (95% CI, 15.9% to 22.0%). The risk was highest among children and was 20.3% (95% CI, 16.4% to 24.9%) for influenza A and 15.9% (95% CI, 11.8% to 21.0%) for influenza B. The overall estimated VE for preventing secondary infections among unvaccinated household contacts was 21.0% (95% CI, 1.4% to 36.7%) and varied by type; estimated VE against influenza A was 5.0% (95% CI, -22.3% to 26.3%) and 56.4% (95% CI, 30.1% to 72.8%) against influenza B.
Conclusions and relevance: After influenza was introduced into households, the risk of secondary influenza among unvaccinated household contacts was approximately 15% to 20%, and highest among children. Estimated VE varied by influenza type, with demonstrated protection against influenza B virus infection.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical