More vs Less Frequent Follow-Up Testing and 10-Year Mortality in Patients With Stage II or III Colorectal Cancer: Secondary Analysis of the COLOFOL Randomized Clinical Trial
- PMID: 39570590
- PMCID: PMC11582930
- DOI: 10.1001/jamanetworkopen.2024.46243
More vs Less Frequent Follow-Up Testing and 10-Year Mortality in Patients With Stage II or III Colorectal Cancer: Secondary Analysis of the COLOFOL Randomized Clinical Trial
Abstract
Importance: Although intensive follow-up of patients after curative surgery for colorectal cancer is common in clinical practice, evidence for a long-term survival benefit of more frequent testing is limited.
Objective: To examine overall and colorectal cancer-specific mortality rates in patients with stage II or III colorectal cancer who underwent curative surgery and underwent high-frequency or low-frequency follow-up testing.
Design, setting, and participants: This randomized clinical trial with posttrial prespecified follow-up was performed in 23 centers in Sweden and Denmark. The original study enrolled 2509 patients with stage II or III colorectal cancer from Sweden, Denmark, and Uruguay (1 center) who received treatment from January 1, 2006, through December 31, 2010, and were followed up for up to 5 years. The participants from Sweden and Denmark were then followed up for 10 years through population-based health registries. The 53 patients from Uruguay were not included in the posttrial follow-up. Statistical analysis was performed from March to June 2024.
Interventions: Patients were randomly allocated to follow-up testing with computed tomography (CT) scans and serum carcinoembryonic antigen (CEA) screening at 6, 12, 18, 24, and 36 months after surgery (high-frequency group; 1227 patients), or at 12 and 36 months after surgery (low-frequency group, 1229 patients).
Main outcomes and measures: The outcomes were 10-year overall mortality and colorectal cancer-specific mortality rates. Both intention-to-treat and per-protocol analyses were performed.
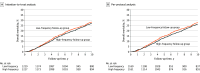
Results: Of the 2555 patients who were randomly allocated, 2509 were included in the intention-to-treat analysis, of whom 2456 (97.9%) were included in this posttrial analysis (median age, 65 years [IQR, 59-70 years]; 1355 male patients [55.2%]). The 10-year overall mortality rate for the high-frequency group was 27.1% (333 of 1227; 95% CI, 24.7%-29.7%) compared with 28.4% (349 of 1229; 95% CI, 26.0%-31.0%) in the low-frequency group (risk difference, 1.3% [95% CI, -2.3% to 4.8%]). The 10-year colorectal cancer-specific mortality rate in the high-frequency group was 15.6% (191 of 1227; 95% CI, 13.6%-17.7%) compared with 16.0% (196 of 1229; 95% CI, 14.0%-18.1%) in the low-frequency group (risk difference, 0.4% [95% CI, -2.5% to 3.3%]). The same pattern resulted from the per-protocol analysis.
Conclusions and relevance: Among patients with stage II or III colorectal cancer, more frequent follow-up testing with CT scans and CEA testing did not result in a significant reduction in 10-year overall mortality or colorectal cancer-specific mortality. The results of this trial should be considered as the evidence base for updating clinical guidelines.
Trial registration: ClinicalTrials.gov Identifier: NCT00225641.
Conflict of interest statement
Figures
References
-
- World Health Organization. Colorectal cancer. 2024. Accessed April 15, 2024. https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer?gad_s...
-
- International Agency for Research on Cancer. Colorectal cancer. 2024. Accessed April 14, 2024. https://www.iarc.who.int/cancer-type/colorectal-cancer/
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical