Diabetes Renders Photoreceptors Susceptible to Retinal Ischemia-Reperfusion Injury
- PMID: 39570639
- PMCID: PMC11585066
- DOI: 10.1167/iovs.65.13.46
Diabetes Renders Photoreceptors Susceptible to Retinal Ischemia-Reperfusion Injury
Abstract
Purpose: Studies have suggested that photoreceptors (PR) are altered by diabetes, contributing to diabetic retinopathy (DR) pathology. Here, we explored the effect of diabetes on retinal ischemic injury.
Methods: Retinal ischemia-reperfusion (IR) injury was caused by elevation of intraocular pressure in 10-week-old BKS db/db type 2 diabetes mellitus (T2DM) mice or C57BL/6J mice at 4 or 12 weeks after streptozotocin (STZ)-induced type 1 diabetes mellitus (T1DM), and respective nondiabetic controls. Retinal neurodegeneration was evaluated by retinal layer thinning, TUNEL staining, and neuron loss. Vascular permeability was evaluated as retinal accumulation of circulating fluorescent albumin. The effects of pretreatment with a sodium-glucose co-transporter (SGLT1/2) inhibitor, phlorizin, were examined.
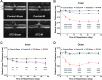
Results: Nondiabetic control mice exhibited no significant outer retinal layer thinning or PR loss after IR injury. In contrast, db/db mice exhibited significant outer retina thinning (49%, P < 0.0001), loss of PR nuclei (45%, P < 0.05) and inner segment (IS) length decline (45%, P < 0.0001). STZ-induced diabetic mice at 4 weeks showed progressive thinning of the outer retina (55%, by 14 days, P < 0.0001) and 4.3-fold greater number of TUNEL+ cells in the outer nuclear layer (ONL) than injured retinas of control mice (P < 0.0001). After 12 weeks of diabetes, the retinas exhibited similar outer layer thinning and PR loss after IR. Diabetes also delayed restoration of the blood-retinal barrier after IR injury. Phlorizin reduced outer retinal layer thinning from 49% to 3% (P < 0.0001).
Conclusions: Diabetes caused PR to become highly susceptible to IR injury. The ability of phlorizin pretreatment to block outer retinal thinning after IR suggests that the effects of diabetes on PR are readily reversible.
Conflict of interest statement
Disclosure:
Figures






References
-
- Agostini H, Abreu F, Baumal CR, et al. .. Faricimab for neovascular age-related macular degeneration and diabetic macular edema: from preclinical studies to phase 3 outcomes [published online ahead of print June 7, 2024]. Graefes Arch Clin Exp Ophthalmol, doi:10.1007/s00417-024-06531-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

