State-level disparities in cervical cancer prevention and outcomes in the United States: a modeling study
- PMID: 39570647
- PMCID: PMC11972673
- DOI: 10.1093/jnci/djae298
State-level disparities in cervical cancer prevention and outcomes in the United States: a modeling study
Abstract
Background: Despite human papillomavirus (HPV) vaccines' availability for over a decade, coverage across the United States varies. Although some states have tried to increase HPV vaccination coverage, most model-based analyses focus on national impacts. We evaluated hypothetical changes in HPV vaccination coverage at the national and state levels for California, New York, and Texas using a mathematical model.
Methods: We developed a new mathematical model of HPV transmission and cervical cancer, creating national- and state-level models, incorporating country- and state-specific vaccination coverage and cervical cancer incidence and mortality. We quantified the national- and state-level impact of increasing HPV vaccination coverage to 80% by 2025 or 2030 on cervical cancer outcomes and the time to elimination defined as less than 4 per 100 000 women.
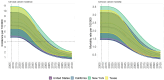
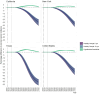
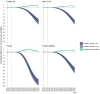
Results: Increasing vaccination coverage to 80% in Texas over 10 years could reduce cervical cancer incidence by 50.9% (95% credible interval [CrI] = 46.6%-56.1%) by 2100, from 1.58 (CrI = 1.19-2.09) to 0.78 (CrI = 0.57-1.02) per 100 000 women. Similarly, New York could see a 27.3% (CrI = 23.9%-31.5%) reduction from 1.43 (CrI = 0.93-2.07) to 1.04 (CrI = 0.66-1.53) per 100 000 women, and California a 24.4% (CrI = 20.0%-30.0%) reduction from 1.01 (CrI = 0.66-1.44) to 0.76 (CrI = 0.50-1.09) per 100 000 women. Achieving 80% coverage in 5 years will provide slightly larger and sooner reductions. If the vaccination coverage levels in 2019 continue, cervical cancer elimination could occur nationally by 2051 (CrI = 2034-2064), but state timelines may vary by decades.
Conclusion: Targeting an HPV vaccination coverage of 80% by 2030 will disproportionately benefit states with low coverage and higher cervical cancer incidence. Geographically focused analyses can better inform priorities.
© The Author(s) 2024. Published by Oxford University Press. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
K.C. is co-principal investigator of an investigator-initiated trial of cervical screening, “Compass,” run by the Australian Centre for Prevention of Cervical Cancer (ACPCC), which is a government-funded not-for-profit charity. Compass receives infrastructure support from the Australian government, and the ACPCC has received equipment and a funding contribution from Roche Molecular Diagnostics, USA. K.C. is also co-principal investigator on a major implementation program, Elimination Partnership for Cervical Cancer in the Indo-PAcific (EPICC) which has received support from the Australian government, the Minderoo Foundation and an equipment donation from Cepheid Inc. All the other authors do not have any conflicts of interest to declare.
Figures
Update of
-
State-level disparities in cervical cancer prevention and outcomes in the U.S.: A modeling study.medRxiv [Preprint]. 2024 Oct 18:2024.06.11.24308795. doi: 10.1101/2024.06.11.24308795. medRxiv. 2024. Update in: J Natl Cancer Inst. 2025 Apr 01;117(4):737-746. doi: 10.1093/jnci/djae298. PMID: 38947042 Free PMC article. Updated. Preprint.
References
-
- International Agency for Research on Cancer (IARC). Cervical Cancer Screening. Vol. 18. IARC; 2022. https://publications.iarc.fr/604
-
- World Health Organization. Cervical cancer elimination initiative. WHO. 2023. Accessed February 28, 2023. https://www.who.int/initiatives/cervical-cancer-elimination-initiative

