Pancreatic CAF-Derived Autotaxin Drives Autocrine CTGF Expression to Modulate Protumorigenic Signaling
- PMID: 39570650
- PMCID: PMC7618285
- DOI: 10.1158/1535-7163.MCT-23-0522
Pancreatic CAF-Derived Autotaxin Drives Autocrine CTGF Expression to Modulate Protumorigenic Signaling
Abstract
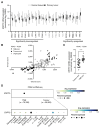
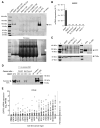
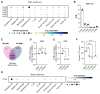
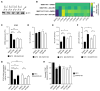
Autotaxin (ATX), encoded by ENPP2, is a clinical target in pancreatic ductal adenocarcinoma (PDAC). ATX catalyzes the production of lysophosphatidic acid (LPA), an important regulator within the tumor microenvironment (TME), yet the protumorigenic action of the ATX/LPA axis in PDAC remains unclear. In this study, by interrogating patient samples and cell line datasets, we show that the PDAC TME, rather than cancer cells, is responsible for the majority of ENPP2 expression and highlight a key role for cancer-associated fibroblast (CAF)-derived ATX in autocrine and paracrine protumorigenic signaling. Using the clinical-stage ATX inhibitor, IOA-289, we identified connective tissue growth factor (CTGF), also known as CCN2, as a downstream mediator of ATX signaling in the PDAC CAF-derived cell line, 0082T. Genetic ablation or pharmacologic inhibition of ATX in 0082T CAFs reduced CTGF secretion via modulation of LPA/LPA receptor signaling. Despite the loss of ATX function, extracellular levels of LPA were paradoxically increased, indicating a role for ATX beyond its enzymatic activity and suggesting a role for its LPA chaperone function in the LPA/LPA receptor signaling in CAFs. As CAFs are the main source for CTGF in the PDAC TME, these findings suggest a role for ATX in promoting a protumorigenic microenvironment via modulation of CAF secretion not only via its LPA-producing activity but also via its LPA chaperone function, providing a potential mechanism for the antitumor effects of ATX inhibition.
©2024 American Association for Cancer Research.
Conflict of interest statement
This study includes results that arise from a collaboration between Cancer Research Horizons (CRH) and iOnctura. RM, FV, AP, LA, ETS, NP, AF, AC, PS, HS and SF are employees of CRH. LZM was a Ph.D. student under the supervision of Michael J. Wakelam and HJS and funded by CRH for this work. MAD, ZJ, GDC, and LV are employees and shareholders of iOnctura.
Figures

References
-
- Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, et al. Identification of Human Plasma Lysophospholipase, D, a Lysophosphatidic Acid-producing Enzyme, as Autotaxin, a Multifunctional Phosphodiesterase*. J Biol Chem. 2002 Oct 18;277(42):39436–42. - PubMed
-
- Kondo M, Ishizawa T, Enooku K, Tokuhara Y, Ohkawa R, Uranbileg B, et al. Increased serum autotaxin levels in hepatocellular carcinoma patients were caused by background liver fibrosis but not by carcinoma. Clin Chim Acta. 2014 Jun 10;433:128–34. - PubMed
-
- Vít O, Petrák J. Autotaxin and Lysophosphatidic Acid Signalling: the Pleiotropic Regulatory Network in Cancer. Folia Biol (Praha) 2024 Apr 7;69(5–6):149–62. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

