HIV-1 Low-Level Viremia Predicts Viral Failure in Participants on Antiretroviral Therapy in the Swiss HIV Cohort Study
- PMID: 39570670
- PMCID: PMC12314502
- DOI: 10.1093/cid/ciae569
HIV-1 Low-Level Viremia Predicts Viral Failure in Participants on Antiretroviral Therapy in the Swiss HIV Cohort Study
Abstract
Background: Most individuals receiving combination antiretroviral therapy (ART) have human immunodeficiency virus (HIV) plasma viral loads below the limit of detection. However, episodes of low-level viremia (LLV) are observed in subsets of individuals, the risk factors and clinical significance of which remain debated.
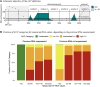
Methods: We included participants enrolled in the Swiss HIV Cohort Study, starting ART between July 1999 and April 2023, with HIV RNA values <200 copies/mL 6 months after ART initiation. Using longitudinally collected data, we applied a time-updated Cox proportional hazards model to determine the association of LLV with the risk of subsequent viral failure, defined as ≥200 copies/mL. LLV was quantified by the time-updated area under the curve (AUC) of HIV RNA values, categorized as undetectable or, based on AUC tertiles, low, intermediate, or high.
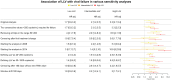
Results: We included 8132 participants with a total of 49 579 person-years of follow-up. The median follow-up time was 4.7 years, and the median number of HIV RNA measurements was 16. Participants had a median age of 38 years, 75.9% were male, 74.4% were white, and 45.9% had HIV-1 subtype B. LLV was associated with an increased risk of subsequent viral failure, with the highest LLV category showing the strongest association (hazard ratio, 3.3 [for comparison with undetectable viral load]) among all included variables, including race/ethnicity, age, and ART.
Conclusions: LLV was strongly associated with risk of subsequent viral failure, even after adjustment for demographic and clinical characteristics, including adherence and treatment regimen. The detection of LLV should prompt appropriate measures to decrease the risk of subsequent viral failure.
Keywords: antiretroviral therapy; longitudinal study; low-level viremia; treatment guidelines; viral failure.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. H. F.'s institution has received educational grants from ViiV, GSK, AbbVie, Gilead, MSD, and Pfizer. A. C. reports unrestricted educational and research grants (paid to the institution) from Gilead Sciences, ViiV Healthcare, and MSD Switzerland. M. C. reports research grants and expert opinion financial compensations paid to the institution from Gilead, MSD, and ViiV. E. B.'s institution reports grants, advisory board fees, and travel grants from Gilead Sciences, MSD, ViiV Healthcare, Pfizer AG, AbbVie, AstraZeneca, Ely Lilly, and Moderna, outside the submitted work. P. S.'s institution has received travel grants, congress and advisory fees from ViiV and Gilead, unrelated to this work. R. D. K. reports grants from the Swiss National Science Foundation, the National Institutes of Health, and Gilead Sciences. H.F.G. has received grants from the Swiss National Science Foundation, the SHCS, the Yvonne Jacob Foundation, the University of Zurich's Clinical Research Priority Program, Zurich Primary HIV Infection, Systems.X, the Bill & Melinda Gates Foundation, National Institutes of Health, Gilead Sciences, ViiV, and Roche; personal fees from Merck, Gilead Sciences, ViiV, Janssen, GSK, Johnson & Johnson, and Novartis for consultancy or data and safety monitoring board membership; and a travel grant from Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Sungkanuparph S, Groger RK, Overton ET, Fraser VJ, Powderly WG. Persistent low-level viraemia and virological failure in HIV-1-infected patients treated with highly active antiretroviral therapy. HIV Med 2006; 7:437–41. - PubMed
-
- Boillat-Blanco N, Darling KEA, Schoni-Affolter F, et al. Virological outcome and management of persistent low-level viraemia in HIV-1-infected patients: 11 years of the Swiss HIV Cohort Study. Antivir Ther 2015; 20:165–75. - PubMed
-
- Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 2010; 304:321–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

