Ferroptosis of select skin epithelial cells initiates and maintains chronic systemic immune-mediated psoriatic disease
- PMID: 39570671
- PMCID: PMC11735110
- DOI: 10.1172/JCI183219
Ferroptosis of select skin epithelial cells initiates and maintains chronic systemic immune-mediated psoriatic disease
Abstract
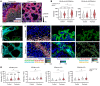
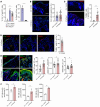
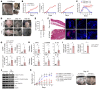
Dysregulations of epithelial-immune interactions frequently culminate in chronic inflammatory diseases of the skin, lungs, kidneys, and gastrointestinal tract. Yet, the intraepithelial processes that initiate and perpetuate inflammation in these organs are poorly understood. Here, by utilizing redox lipidomics we identified ferroptosis-associated peroxidation of polyunsaturated phosphatidylethanolamines in the epithelia of patients with asthma, cystic fibrosis, psoriasis, and renal failure. Focusing on psoriasis as a disease model, we used high-resolution mass spectrometry imaging and identified keratin 14-expressing (K14-expressing) keratinocytes executing a ferroptotic death program in human psoriatic skin. Psoriatic phenotype with characteristic Th1/Th17 skin and extracutaneous immune responses was initiated and maintained in a murine model designed to actuate ferroptosis in a fraction of K14+ glutathione peroxidase 4-deficient (Gpx4-deficient) epidermal keratinocytes. Importantly, an antiferroptotic agent, liproxstatin-1, was as effective as clinically relevant biological IL-12/IL-23/TNF-α-targeting therapies or the depletion of T cells in completely abrogating molecular, biochemical, and morphological features of psoriasis. As ferroptosis in select epidermal keratinocytes triggers and sustains a pathological psoriatic multiorgan inflammatory circuit, we suggest that strategies targeting ferroptosis or its causes may be effective in preventing or ameliorating a variety of chronic inflammatory diseases.
Keywords: Autoimmune diseases; Cell stress; Dermatology; Inflammation; Skin.
Figures



References
MeSH terms
Substances
Grants and funding
- R01 CA165065/CA/NCI NIH HHS/United States
- R01 AR067746/AR/NIAMS NIH HHS/United States
- U01 AI156924/AI/NIAID NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- R01 CA266342/CA/NCI NIH HHS/United States
- U01 AI156923/AI/NIAID NIH HHS/United States
- R01 DK070910/DK/NIDDK NIH HHS/United States
- R01 AI145406/AI/NIAID NIH HHS/United States
- R01 NS061817/NS/NINDS NIH HHS/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
- R01 CA272946/CA/NCI NIH HHS/United States
- R01 CA266529/CA/NCI NIH HHS/United States
- R01 NS076511/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

