Multiomics Analysis Reveals Molecular Changes during Early Progression of Precancerous Lesions to Lung Adenocarcinoma in Never-Smokers
- PMID: 39570802
- PMCID: PMC11786955
- DOI: 10.1158/0008-5472.CAN-24-0821
Multiomics Analysis Reveals Molecular Changes during Early Progression of Precancerous Lesions to Lung Adenocarcinoma in Never-Smokers
Abstract
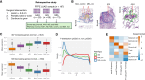
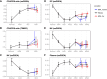
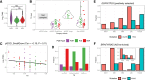
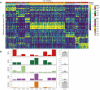
Lung cancer is the most common cause of cancer mortality globally, and the prevalence of lung adenocarcinoma, the most common lung cancer subtype, has increased sharply in East Asia. Early diagnosis leads to better survival rates, but this requires an improved understanding of the molecular changes during early tumorigenesis, particularly in nonsmokers. In this study, we performed whole-exome sequencing and RNA sequencing of samples from 94 East Asian patients with precancerous lesions [25 with atypical adenomatous hyperplasia (AAH); 69 with adenocarcinoma in situ (AIS)] and 73 patients with early invasive lesions [minimally invasive adenocarcinoma (MIA)]. Cellular analysis revealed that the activities of endothelial and stromal cells could be used to categorize tumors into molecular subtypes within pathologically defined types of lesions. The subtypes were linked with the radiologically defined type of lesions and corresponded to immune cell infiltration throughout the early progression of lung adenocarcinoma. Spatial transcriptomic analysis revealed the distribution of epithelial cells, endothelial cells, fibroblasts, and plasma cells within MIA samples. Characterization of the molecular lesion subtypes identified positively selected mutational patterns and suggested that angiogenesis in the late-stage AIS type potentially contributes to tissue invasion of the MIA type. This study offers a resource that may help improve early diagnosis and patient prognosis, and the findings suggest possible approaches for early disease interception. Significance: Integrative analysis of multiomics data revealed coordination between immune and nonimmune cells during early progression of precancerous lesions to lung adenocarcinomas and shed light on the molecular characteristics of clinically defined subtypes.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
Y.-C. Chen reports current employment with Johnson & Johnson, as well as holding shares in the company. H.-M. Wang reports employment with Johnson & Johnson at the time of analysis. M. Pirooznia reports current employment with Johnson & Johnson, as well as holding shares in the company. R. Yang reports other support from Johnson & Johnson outside the submitted work. J.-Y. Shih reports grants from National Science and Technology Council during the conduct of the study, as well as grants from Roche and personal fees from AbbVie, ACT Genomics, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly and Company, Guardant Health, Illumina, Janssen, Lotus, Merck Sharp & Dohme, Merck, Ono Pharmaceutical, Orient EuroPharma, Pfizer, Roche, Takeda, TSH Biopharm, and TTY Biopharm outside the submitted work. No disclosures were reported by the other authors.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Myers DJ, Wallen JM. Lung adenocarcinoma. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. - PubMed
-
- Sakurai H, Maeshima A, Watanabe S-i, Suzuki K, Tsuchiya R, Maeshima AM, et al. . Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol 2004;28:198–206. - PubMed
-
- Noguchi M. Stepwise progression of pulmonary adenocarcinoma–clinical and molecular implications. Cancer Metastasis Rev 2010;29:15–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

