Cardiac effects of OPA1 protein promotion in a transgenic animal model
- PMID: 39570915
- PMCID: PMC11581344
- DOI: 10.1371/journal.pone.0310394
Cardiac effects of OPA1 protein promotion in a transgenic animal model
Abstract
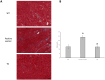
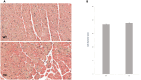
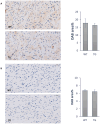
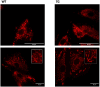
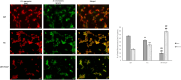
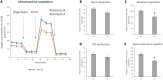
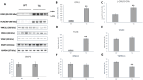
Mitochondria form a dynamic network in cells, regulated by the balance between mitochondrial fusion and fission. The inhibition of mitochondrial fission can have positive effects in acute ischemic/reperfusion injury models by preventing the fall in mitochondrial membrane potential associated with fission processes. However, inhibition of fission in chronic models is disadvantageous because it obstructs the elimination of damaged mitochondrial fragments. OPA1, in view of previous results, is a possible therapeutic target as a fusion promoter and structure stabilizer protein. We used transgenic mice in which the OMA1 cleavage sites of OPA1 were deleted. This resulted in a higher representation of L-OPA1 compared to S-OPA1. After genotyping and model validation, all animals were examined by echocardiograph on two occasions, at weeks 11 and 36. Histological samples were taken from hearts to examine mitochondrial morphology and structure remodeling. The signaling pathways related to mitochondrial dynamic processes were evaluated. Cardiomyocytes were isolated from neonatal mice to determine the efficiency of mitochondrial respiration using the SeaHorse assay method. OPA1 protein promotion has a negative effect on systolic function during aging. We confirmed that volume overload and ventricular remodeling did not manifest. The reason behind the loss of pump function might be, at least partly, due to the energy deficit caused by mitochondrial respiratory failure and damage in mitochondrial quality control pathways.
Copyright: © 2024 Bruszt et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: one of the coauthors, Ferenc Gallyas is a section editor of the journal.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

