Long-term cognitive effects of menopausal hormone therapy: Findings from the KEEPS Continuation Study
- PMID: 39570992
- PMCID: PMC11581397
- DOI: 10.1371/journal.pmed.1004435
Long-term cognitive effects of menopausal hormone therapy: Findings from the KEEPS Continuation Study
Abstract
Background: Findings from Kronos Early Estrogen Prevention Study (KEEPS)-Cog trial suggested no cognitive benefit or harm after 48 months of menopausal hormone therapy (mHT) initiated within 3 years of final menstrual period. To clarify the long-term effects of mHT initiated in early postmenopause, the observational KEEPS Continuation Study reevaluated cognition, mood, and neuroimaging effects in participants enrolled in the KEEPS-Cog and its parent study the KEEPS approximately 10 years after trial completion. We hypothesized that women randomized to transdermal estradiol (tE2) during early postmenopause would show cognitive benefits, while oral conjugated equine estrogens (oCEE) would show no effect, compared to placebo over the 10 years following randomization in the KEEPS trial.
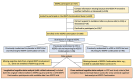
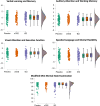
Methods and findings: The KEEPS-Cog (2005-2008) was an ancillary study to the KEEPS (NCT00154180), in which participants were randomized into 3 groups: oCEE (Premarin, 0.45 mg/d), tE2 (Climara, 50 μg/d) both with micronized progesterone (Prometrium, 200 mg/d for 12 d/mo) or placebo pills and patch for 48 months. KEEPS Continuation (2017-2022), an observational, longitudinal cohort study of KEEPS clinical trial, involved recontacting KEEPS participants approximately 10 years after the completion of the 4-year clinical trial to attend in-person research visits. Seven of the original 9 sites participated in the KEEPS Continuation, resulting in 622 women of original 727 being invited to return for a visit, with 299 enrolling across the 7 sites. KEEPS Continuation participants repeated the original KEEPS-Cog test battery which was analyzed using 4 cognitive factor scores and a global cognitive score. Cognitive data from both KEEPS and KEEPS Continuation were available for 275 participants. Latent growth models (LGMs) assessed whether baseline cognition and cognitive changes during KEEPS predicted cognitive performance at follow-up, and whether mHT randomization modified these relationships, adjusting for covariates. Similar health characteristics were observed at KEEPS randomization for KEEPS Continuation participants and nonparticipants (i.e., women not returning for the KEEPS Continuation). The LGM revealed significant associations between intercepts and slopes for cognitive performance across almost all domains, indicating that cognitive factor scores changed over time. Tests assessing the effects of mHT allocation on cognitive slopes during the KEEPS and across all years of follow-up including the KEEPS Continuation visit were all statistically nonsignificant. The KEEPS Continuation study found no long-term cognitive effects of mHT, with baseline cognition and changes during KEEPS being the strongest predictors of later performance. Cross-sectional comparisons confirmed that participants assigned to mHT in KEEPS (oCEE and tE2 groups) performed similarly on cognitive measures to those randomized to placebo, approximately 10 years after completion of the randomized treatments. These findings suggest that mHT poses no long-term cognitive harm; conversely, it provides no cognitive benefit or protective effects against cognitive decline.
Conclusions: In these KEEPS Continuation analyses, there were no long-term cognitive effects of short-term exposure to mHT started in early menopause versus placebo. These data provide reassurance about the long-term neurocognitive safety of mHT for symptom management in healthy, recently postmenopausal women, while also suggesting that mHT does not improve or preserve cognitive function in this population.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
K.K. served on the data safety monitoring board for Pfizer Inc. and Takeda Global Research & Development Center, Inc. She received research support from Avid. MM-A received consulting fees from the Biomedical Research Alliance of New York. Radiopharmaceuticals, Eli Lilly. She consults for Biogen.
Figures

Comment in
-
A consideration of the potential benefits and harms of menopausal hormone treatment.PLoS Med. 2025 Apr 10;22(4):e1004567. doi: 10.1371/journal.pmed.1004567. eCollection 2025 Apr. PLoS Med. 2025. PMID: 40208881 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

