Normal Bone Matrix Mineralization but Altered Growth Plate Morphology in the LmnaG609G/G609G Mouse Model of Progeria
- PMID: 39571160
- PMCID: PMC12339082
- DOI: 10.14336/AD.2024.1094
Normal Bone Matrix Mineralization but Altered Growth Plate Morphology in the LmnaG609G/G609G Mouse Model of Progeria
Abstract
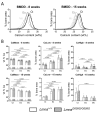
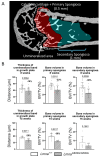
Hutchison-Gilford progeria syndrome (HGPS) is a rare genetic disease caused by a mutation in LMNA, the gene encoding A-type lamins, leading to premature aging with severely reduced life span. HGPS is characterized by growth deficiency, subcutaneous fat and muscle issues, wrinkled skin, alopecia, and atherosclerosis. Patients also develop a bone phenotype with reduced bone mineral density, osteolysis and striking demineralization of long bones. To further clarify the tissue modifications in HGPS, we characterized bone mineralization in the LmnaG609G/G609G progeria mouse model. Femurs from 8-week-old mice and humeri from 15-week-old mice were analyzed using quantitative backscattered electron imaging to assess bone mineralization density distribution, osteocyte lacunae sections and structural bone histomorphometry. Tissue sections were stained with Giemsa and Goldner trichrome for histologic evaluation. Bone tissue from Lmna+/+ and LmnaG609G/G609G mice had similar mineral content at 3 different bone sites with specific tissue ages. The osteocyte lacunae features were not statistically different, but more empty lacunae were found in LmnaG609G/G609G at both animal ages. Bone histomorphometry and histology demonstrated decreased bone volume per tissue volume in primary (8W: -23%, p=0.001; 15W: -38%, p=0.002) and secondary spongiosa (8W: -36%, p=0.001; 15W: -49 %, ns), as well as growth plate dysplasia with thinner unmineralized resting and proliferative zones in the LmnaG609G/G609G mice versus controls (8W: -18%, p=0.006; 15W: -25%, p=0.001). Overall, the LmnaG609G/G609G mouse develops chondrodysplasia with reduced trabecular bone volume. Mineral content findings at several tissue sites and ages suggest that bone dysplasia results from impaired bone formation with normal bone turnover.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hennekam RC (2006). Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A, 140:2603-2624. - PubMed
-
- Ullrich NJ, Gordon LB (2015). Hutchinson-Gilford progeria syndrome. Handb Clin Neurol, 132:249-264. - PubMed
-
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. (2003). Lamin a truncation in Hutchinson-Gilford progeria. Science, 300:2055. - PubMed
-
- Machiels BM, Zorenc AH, Endert JM, Kuijpers HJ, van Eys GJ, Ramaekers FC, et al. (1996). An alternative splicing product of the lamin A/C gene lacks exon 10. J Biol Chem, 271:9249-9253. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
