Single-Cell Multi-Omics Analysis of In Vitro Post-Ovulatory-Aged Oocytes Revealed Aging-Dependent Protein Degradation
- PMID: 39571909
- PMCID: PMC11728983
- DOI: 10.1016/j.mcpro.2024.100882
Single-Cell Multi-Omics Analysis of In Vitro Post-Ovulatory-Aged Oocytes Revealed Aging-Dependent Protein Degradation
Abstract
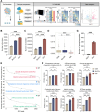
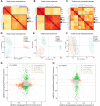
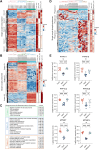
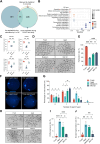
Once ovulated, the oocyte has to be fertilized in a short time window or it will undergo post-ovulation aging (POA), whose underlying mechanisms are still not elucidated. Here, we optimized single-cell proteomics methods and performed single-cell transcriptomic, proteomic, and phosphoproteomic analysis of fresh, POA, and melatonin-treated POA oocytes. POA oocytes showed downregulation of most differentially expressed proteins, with little correlation with mRNA expression, and the protein changes can be rescued by melatonin treatment. MG132 treatment rescued the decreased fertilization and polyspermy rates and upregulated fragmentation and parthenogenesis rates of POA oocytes. MG132-treated oocytes displayed health status at proteome, phosphoproteome, and fertilization ability similar to fresh oocytes, suggesting that protein stabilization might be the underlying mechanism for melatonin to rescue POA. The important roles of proteasome-mediated protein degradation during oocyte POA revealed by single-cell multi-omics analyses offer new perspectives for increasing oocyte quality during POA and improving assisted reproduction technologies.
Keywords: melatonin; mouse; multi-omics; oocyte; post-ovulation aging; single cell.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

