Reaction-Type-Dependent Behavior of Redox-Hopping in MOFs─Does Charge Transport Have a Preferred Direction?
- PMID: 39572009
- PMCID: PMC11626502
- DOI: 10.1021/acs.jpclett.4c01674
Reaction-Type-Dependent Behavior of Redox-Hopping in MOFs─Does Charge Transport Have a Preferred Direction?
Abstract
Redox hopping is the primary method of electron transport through redox-active metal-organic frameworks (MOFs). While redox hopping adequately supports the electrocatalytic application of MOFs, the fundamental understandings guiding the design of redox hopping MOFs remain nascent. In this study, we probe the rate of electron and hole transport through a singular MOF scaffold to determine whether the properties of the MOF promote the transport of one carrier over the other. A redox center, [RuII(bpy)2(bpy-COOH)]2+, where bpy = 2,2'-bipyridine and bpy-COOH = 4-carboxy-2,2'-bipyridine, was anchored within NU-1000. The electron hopping coefficients (De) and ion diffusion coefficients (Di) were calculated via chronoamperometry and application of the Scholz model. We found that electrons transport more rapidly than holes in the studied MOF. Interestingly, the correlation between De and self-exchange rate built in previous research predicted reversely. The contradicting result indicates that spacing between the molecular moieties involved in a particular hopping process dominates the response.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




 vs
vs  Scholz plots for reduction (c) and oxidation
(d) used to determine tref.
Scholz plots for reduction (c) and oxidation
(d) used to determine tref.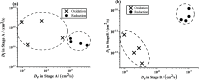

Similar articles
-
Charge Transport in Zirconium-Based Metal-Organic Frameworks.Acc Chem Res. 2020 Jun 16;53(6):1187-1195. doi: 10.1021/acs.accounts.0c00106. Epub 2020 May 13. Acc Chem Res. 2020. PMID: 32401008
-
The Molecular Nature of Redox-Conductive Metal-Organic Frameworks.Acc Chem Res. 2024 Oct 1;57(19):2836-2846. doi: 10.1021/acs.accounts.4c00430. Epub 2024 Sep 17. Acc Chem Res. 2024. PMID: 39288193 Free PMC article.
-
The role of redox hopping in metal-organic framework electrocatalysis.Chem Commun (Camb). 2018 Jun 21;54(51):6965-6974. doi: 10.1039/c8cc01664j. Chem Commun (Camb). 2018. PMID: 29809219
-
Redox Hopping in Metal-Organic Frameworks through the Lens of the Scholz Model.J Phys Chem Lett. 2023 Nov 30;14(47):10700-10709. doi: 10.1021/acs.jpclett.3c02641. Epub 2023 Nov 21. J Phys Chem Lett. 2023. PMID: 37988693 Review.
-
Functional metal-organic frameworks derived electrode materials for electrochemical energy storage: a review.Chem Commun (Camb). 2024 Nov 12;60(91):13292-13313. doi: 10.1039/d4cc04086d. Chem Commun (Camb). 2024. PMID: 39465622 Review.
Cited by
-
Charge Transfer and Recombination Pathways through Fullerene Guests in Porphyrin-Based MOFs.J Phys Chem C Nanomater Interfaces. 2025 Apr 23;129(17):8215-8227. doi: 10.1021/acs.jpcc.5c00161. eCollection 2025 May 1. J Phys Chem C Nanomater Interfaces. 2025. PMID: 40786243 Free PMC article.
References
-
- Duan J.; Goswami S.; Patwardhan S.; Hupp J. T. Does the Mode of Metal–Organic Framework/Electrode Adhesion Determine Rates for Redox-Hopping-Based Charge Transport within Thin-Film Metal–Organic Frameworks?. J. Phys. Chem. C 2022, 126 (9), 4601–4611. 10.1021/acs.jpcc.1c09812. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

