Animal models of Alzheimer's disease: Current strategies and new directions
- PMID: 39572020
- PMCID: PMC11668949
- DOI: 10.24272/j.issn.2095-8137.2024.274
Animal models of Alzheimer's disease: Current strategies and new directions
Abstract
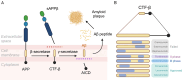
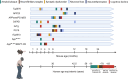
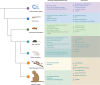
Animal models constructed using pathogenic factors have significantly advanced drug development for Alzheimer's disease (AD). These predominantly transgenic models, mainly in mice, replicate pathological phenotypes through gene mutations associated with familial AD cases, thus serving as vital tools for assessing drug efficacy and for performing mechanistic studies. However, the species-specific differences and complex, heterogeneous nature of AD etiology pose considerable challenges for the translatability of these animal models, limiting their utility in drug development. This review offers a comprehensive analysis of widely employed rodent (mice and rats) and non-rodent models ( Danio rerio (zebrafish), Drosophila melanogaster, and Caenorhabditis elegans), detailing their phenotypic features and specific research applications. This review also examines the limitations inherent in these models and introduces various strategies for expanding AD modeling across diverse species, emphasizing recent advancement in non-human primates (NHPs) as valuable models. Furthermore, potential insights from the integration of innovative technologies in AD research are discussed, while providing valuable perspectives on the future development of AD animal models.
基于致病因素构建的动物模型推动了阿尔茨海默病(Alzheimer’s disease, AD)药物的开发。目前,小鼠是AD转基因模型最主要的模式动物,该方法是通过引入在家族性病例中发现的基因突变来再现病理表型。这些模型不仅为药物疗效评估提供了平台,也促进了机制研究。然而,由于物种差异,以及AD病因的高度异质性,这些动物模型也存在固有隐患,影响了药物开发。在该文中,我们全面概述了广泛应用的啮齿动物(小鼠和大鼠)及非啮齿动物(斑马鱼、果蝇和秀丽隐杆线虫)模型的表型特征及其相应应用。通过分析这些模型固有的局限性,我们现有的多种AD建模策略,并特别强调了非人灵长类(Non-human primates, NHP)模型的进展。此外,我们讨论了创新技术在AD研究中的整合可能带来的深远启示,同时为未来AD动物模型的发展提供了有价值的观点。.
Keywords: Alzheimer’s disease; Animal models; Aβ; Non-human primates; Tau.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Experimental modeling of Alzheimer's disease: Translational lessons from cross-taxon analyses.Alzheimers Dement. 2025 May;21(5):e70273. doi: 10.1002/alz.70273. Alzheimers Dement. 2025. PMID: 40420360 Free PMC article. Review.
-
Research on Alzheimer's Disease (AD) Involving the Use of In vivo and In vitro Models and Mechanisms.Cent Nerv Syst Agents Med Chem. 2025;25(2):123-142. doi: 10.2174/0118715249293642240522054929. Cent Nerv Syst Agents Med Chem. 2025. PMID: 38803173 Review.
-
Overview of Transgenic Mouse Models for Alzheimer's Disease.Curr Protoc Neurosci. 2019 Sep;89(1):e81. doi: 10.1002/cpns.81. Curr Protoc Neurosci. 2019. PMID: 31532917 Review.
-
Animal models of Alzheimer's disease: therapeutic implications.J Alzheimers Dis. 2008 Dec;15(4):507-21. doi: 10.3233/jad-2008-15401. J Alzheimers Dis. 2008. PMID: 19096153 Review.
-
Modeling Alzheimer's Disease: A Review of Gene-Modified and Induced Animal Models, Complex Cell Culture Models, and Computational Modeling.Brain Sci. 2025 May 5;15(5):486. doi: 10.3390/brainsci15050486. Brain Sci. 2025. PMID: 40426657 Free PMC article. Review.
Cited by
-
Novel mouse model of Alzheimer's disease exhibits pathology through synergistic interactions among amyloid-β, tau, and reactive astrogliosis.Zool Res. 2025 Jan 18;46(1):41-53. doi: 10.24272/j.issn.2095-8137.2024.257. Zool Res. 2025. PMID: 39757019 Free PMC article.
-
Early transcriptional and cellular abnormalities in choroid plexus of a mouse model of Alzheimer's disease.Mol Neurodegener. 2025 May 31;20(1):62. doi: 10.1186/s13024-025-00853-w. Mol Neurodegener. 2025. PMID: 40450296 Free PMC article.
-
Behavioral and pathological characteristics of 5xFAD female mice in the early stage.Sci Rep. 2025 Feb 26;15(1):6924. doi: 10.1038/s41598-025-90335-2. Sci Rep. 2025. PMID: 40011556 Free PMC article.
-
Beyond Transgenic Mice: Emerging Models and Translational Strategies in Alzheimer's Disease.Int J Mol Sci. 2025 Jun 10;26(12):5541. doi: 10.3390/ijms26125541. Int J Mol Sci. 2025. PMID: 40565005 Free PMC article. Review.
-
The efficacy and pharmacological mechanism of Guilingji to prevent Alzheimer's disease.Alzheimers Res Ther. 2025 Jul 14;17(1):157. doi: 10.1186/s13195-025-01790-y. Alzheimers Res Ther. 2025. PMID: 40660408 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
