Striatal Interneuron Imbalance in a Valproic Acid-Induced Model of Autism in Rodents Is Accompanied by Atypical Somatosensory Processing
- PMID: 39572246
- PMCID: PMC11653103
- DOI: 10.1523/ENEURO.0326-24.2024
Striatal Interneuron Imbalance in a Valproic Acid-Induced Model of Autism in Rodents Is Accompanied by Atypical Somatosensory Processing
Abstract
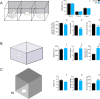
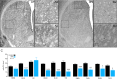
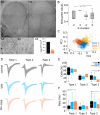
Autism spectrum disorder (ASD) is characterized by deficits in social interaction and communication, cognitive rigidity, and atypical sensory processing. Recent studies suggest that the basal ganglia, specifically the striatum (NSt), plays an important role in ASD. While striatal interneurons, including cholinergic (ChAT+) and parvalbumin-positive (PV+) GABAergic neurons, have been described to be altered in animal models of ASD, their specific contribution remains elusive. Here, we combined behavioral, anatomical, and electrophysiological quantifications to explore if interneuron balance could be implicated in atypical sensory processing in cortical and striatal somatosensory regions of rats subjected to a valproic acid (VPA) model of ASD. We found that VPA animals showed a significant decrease in the number of ChAT+ and PV+ cells in multiple regions (including the sensorimotor region) of the NSt. We also observed significantly different sensory-evoked responses at the single-neuron and population levels in both striatal and cortical regions, as well as corticostriatal interactions. Therefore, selective elimination of striatal PV+ neurons only partially recapitulated the effects of VPA, indicating that the mechanisms behind the VPA phenotype are much more complex than the elimination of a particular neural subpopulation. Our results indicate that VPA exposure induced significant histological changes in ChAT+ and PV+ cells accompanied by atypical sensory-evoked corticostriatal population dynamics that could partially explain the sensory processing differences associated with ASD.
Keywords: autism spectrum disorder; sensory processing; striatal interneurons; valproic acid.
Copyright © 2024 Ibáñez-Sandoval et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







Similar articles
-
Prenatal Valproate Exposure Differentially Affects Parvalbumin-Expressing Neurons and Related Circuits in the Cortex and Striatum of Mice.Front Mol Neurosci. 2016 Dec 21;9:150. doi: 10.3389/fnmol.2016.00150. eCollection 2016. Front Mol Neurosci. 2016. PMID: 28066177 Free PMC article.
-
Agmatine ameliorates valproic acid-induced depletion of parvalbumin-positive neuron.Int J Dev Neurosci. 2024 Apr;84(2):134-142. doi: 10.1002/jdn.10314. Epub 2024 Feb 2. Int J Dev Neurosci. 2024. PMID: 38304999
-
Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism.Mol Brain. 2016 Jan 27;9:10. doi: 10.1186/s13041-016-0192-8. Mol Brain. 2016. PMID: 26819149 Free PMC article.
-
The Role of Parvalbumin Interneurons in Autism Spectrum Disorder.J Neurosci Res. 2024 Oct;102(10):e25391. doi: 10.1002/jnr.25391. J Neurosci Res. 2024. PMID: 39400385 Review.
-
Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder.Neuropharmacology. 2019 Nov 15;159:107477. doi: 10.1016/j.neuropharm.2018.12.024. Epub 2019 Jan 9. Neuropharmacology. 2019. PMID: 30639388 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
