Effect of intraperitoneal ropivacaine during and after cytoreductive surgery on time-interval to adjuvant chemotherapy in advanced ovarian cancer: a randomised, double-blind phase III trial
- PMID: 39572271
- PMCID: PMC11867074
- DOI: 10.1016/j.bja.2024.10.015
Effect of intraperitoneal ropivacaine during and after cytoreductive surgery on time-interval to adjuvant chemotherapy in advanced ovarian cancer: a randomised, double-blind phase III trial
Abstract
Background: In a previous phase II trial, intraperitoneal local anaesthetics shortened the time interval between surgery and adjuvant chemotherapy, an endpoint associated with improved survival in advanced ovarian cancer. Our objective was to test this in a phase III trial.
Methods: A double-blind, phase III parallel superiority trial was conducted at two university hospitals in Sweden, within a public and centralised healthcare system. Women >18 yr with advanced ovarian cancer scheduled for cytoreductive surgery, an ASA physical status of 1-3 with no speech/language issues, were eligible. Participants were randomly assigned using a central computerised system to receive either ropivacaine 0.2% or saline 0.9% (placebo) intraperitoneally during and after surgery. The primary endpoint was time to return to intended oncologic therapy (RIOT), analysed using t-test and linear regression adjusted for centre.
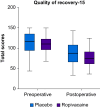
Results: Of the 225 women randomised between August 2020 and December 2023 (ropivacaine n=113; placebo n=112), 175 were included in the modified intention-to-treat analysis (ropivacaine n=86; placebo n=89). Median age: ropivacaine group 64 yr (56-73 yr), placebo group: 66 yr (57-74 yr). The mean RIOT in the ropivacaine group was 26.5 days vs 25.8 days in the placebo group, with a mean difference of 0.7 days (-2.2 to 3.4 days; P=0.65). Per-protocol analysis of 166 women yielded similar results, mean difference of 0.5 days (-2.4 to 3.4 days; P=0.74) days. There were no differences in short-term recovery or postoperative morbidity.
Conclusion: Intraperitoneal local anaesthetic did not shorten the time to RIOT among women undergoing surgery for advanced ovarian cancer in this trial.
Clinical trial registration: ClinicalTrials.gov (NCT04065009), European Union Clinical Trials Register (2019-003299-38/SE).
Keywords: cytoreductive surgery; intraperitoneal; local anaesthetic; ovarian cancer; return to intended oncologic therapy; ropivacaine; time interval to chemotherapy.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Bristow R.E. K.B., Chi D.S. Informa Healthcare; New York: 2011. Surgery for Ovarian Cancer Principles and Practice.
-
- Norton L., Simon R. The Norton-Simon hypothesis revisited. Cancer Treat Rep. 1986;70:163–169. - PubMed
-
- Bristow R.E., Tomacruz R.S., Armstrong D.K., Trimble E.L., Montz F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. - PubMed
-
- Mahner S., Eulenburg C., Staehle A., et al. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: analysis of prospective randomised phase III trials. Eur J Cancer. 2013;49:142–149. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

