A phase 2 clinical trial of luspatercept in non-transfusion-dependent patients with myelodysplastic syndromes
- PMID: 39572468
- PMCID: PMC11741997
- DOI: 10.1007/s12185-024-03872-3
A phase 2 clinical trial of luspatercept in non-transfusion-dependent patients with myelodysplastic syndromes
Abstract
Luspatercept has shown durable clinical efficacy for the treatment of anemia in transfusion-dependent patients with lower-risk myelodysplastic syndromes (LR-MDS). We report the results of a prespecified primary analysis of a phase 2 trial of luspatercept in non-transfusion-dependent (NTD) Japanese patients with anemia due to LR-MDS. Luspatercept (starting dose 1.0 mg/kg) was administered subcutaneously once every 3 weeks. The primary endpoint was the proportion of patients who achieved hematological improvement-erythroid (HI-E) response (≥ 1.5 g/dL increase in hemoglobin level for 8 weeks) without transfusions within the first 24 weeks of treatment. At the primary analysis data cutoff, 21 patients had been enrolled/treated; 17 and 10 patients had completed 24 and 48 weeks of treatment, respectively. HI-E response occurred within 24 weeks in 10 patients (47.6%; 95% confidence interval, 25.7-70.2; P < 0.0001), which was significantly higher than the predefined threshold (10%). By week 48, HI-E response occurred in 12 patients (57.1%) and 17 patients (81.0%) remained NTD. Luspatercept was well tolerated. Three patients (14.3%) had grade 3-4 treatment-related treatment-emergent adverse events. Luspatercept resulted in statistically and clinically significant improvements in hemoglobin levels, and may help delay the need for transfusions in NTD patients with LR-MDS.
Keywords: Anemia; Low risk; Luspatercept; Myelodysplastic syndromes; Non-transfusion dependent.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: H.K. received honoraria or lecture fees from Bristol Myers Squibb, AbbVie GK, Asahi Kasei Pharma, AstraZeneca K.K., Beckton Dickinson K.K., Chugai Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., Janssen Pharmaceutical K.K., Japan Blood Products Organization, Kissei Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Nippon Shinyaku Co., Ltd., Novartis Pharma K.K., Novo Nordisk Pharma Japan, Ono Pharmaceutical Co., Ltd., Sanofi K.K, and Takeda Pharmaceutical Co., Ltd. H.Iw. received honoraria or lecture fees from Astra Zeneca, AbbVie, Ono Pharmaceuticals, Janssen Pharmaceuticals, Sanofi, Daiichi Sankyo, Chugai Pharmaceuticals, and Takeda Pharmaceuticals; manuscript fees for promotional materials from SymBio Pharmaceuticals; and research funds for medical research from Kyowa Kirin. H.Ii. received honoraria or lecture fees from Novartis, Janssen Pharmaceuticals, Takeda Pharmaceuticals, Chugai Pharmaceuticals, Sanofi, Otsuka Pharmaceuticals, and AbbVie; and research funds for medical research from Nippon Kayaku and Chugai Pharmaceuticals. T.J. received honoraria or lecture fees from Eisai; and research funds for medical research from Bristol Myers Squibb. Y.M. received honoraria or lecture fees from Nippon Shinyaku; and a subsidy/donation from Eisai for presenting academic courses. Y.N. received research funds for medical research from Astellas Pharma, AbbVie, Celgene, Novartis Pharma, and Bristol Myers Squibb. K.O. has received research funds for medical research from Celgene. T.S. received honoraria or lecture fees from Alexion Pharmaceuticals, Novartis Pharma, Kyowa Kirin, Chugai Pharmaceuticals, and Bristol Myers Squibb. Y.T. received honoraria or lecture fees from Janssen Pharmaceuticals, Sanofi, and Chugai Pharmaceuticals. K.U. received honoraria or lecture fees from Novartis Pharma; and research funds for medical research from Astellas Pharma, AbbVie, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol Myers Squibb, and Otsuka Pharmaceuticals. H.Y. owns stock or options in Bristol Myers Squibb and Merck, and is an employee of Bristol Myers Squibb. J.H. is an employee of Bristol Myers Squibb. S.M. owns stock or options in, and is an employee of, Bristol Myers Squibb. M.N. owns stock or options in, and is an employee of, Bristol Myers Squibb. M.S. is an employee of Bristol Myers Squibb. K.M. received research funds for medical research from Janssen Pharmaceutical, Bristol Myers Squibb, and Sanofi. T.F., A.S., S.K., K.S., and A.Y. have no conflicts of interest to disclose.
Figures
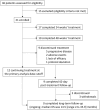
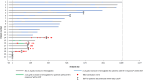

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

