Astrocytic inhibition of lateral septal neurons promotes diverse stress responses
- PMID: 39572547
- PMCID: PMC11582824
- DOI: 10.1038/s41467-024-54376-x
Astrocytic inhibition of lateral septal neurons promotes diverse stress responses
Abstract
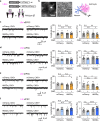
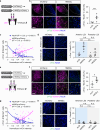
Inhibitory neuronal circuits within the lateral septum (LS) play a key role in regulating mood and stress responses. Even though glial cells can modulate these circuits, the impact of astrocytes on LS neural circuits and their functional interactions remains largely unexplored. Here, we demonstrate that astrocytes exhibit increased intracellular Ca²⁺ levels in response to aversive sensory and social stimuli in both male and female mice. This astrocytic Ca²⁺ elevation inhibits neighboring LS neurons by reducing excitatory synaptic transmissions through A1R-mediated signaling in both the dorsal (LSd) and intermediate LS (LSi) and enhancing inhibitory synaptic transmission via A2AR-mediated signaling in the LSi. At the same time, astrocytes reduce inhibitory tone on distant LS neurons. In the LSd, astrocytes promote social avoidance and anxiety, as well as increased heart rate in socially stressed male mice. In contrast, astrocytes in the LSi contribute to elevated heart rate and heightened blood corticosterone levels in unstressed male mice. These results suggest that the dynamic interactions between astrocytes and neurons within the LS modulate physiological and behavioral responses to stressful experiences.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

