Enantioselective adsorption on chiral ceramics with medium entropy
- PMID: 39572550
- PMCID: PMC11582819
- DOI: 10.1038/s41467-024-54414-8
Enantioselective adsorption on chiral ceramics with medium entropy
Abstract
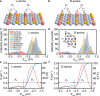
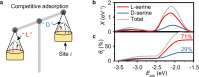
Chiral metal surfaces provide an environment for enantioselective adsorption in various processes such as asymmetric catalysis, chiral recognition, and separation. However, they often suffer from limitations such as reduced enantioselectivity caused by kink coalescence and atomic roughness. Here, we present an approach using medium-entropy ceramic (MEC), specifically (CrMoTa)Si2 with a C40 hexagonal crystal structure, which overcomes the trade-off between thermal stability and enantioselectivity. Experimental confirmation is provided by employing quartz crystal microbalance (QCM), where the electrode is coated with MEC films using non-reactive magnetron sputtering technology. The chiral nature is verified through transmission electron microscopy and circular dichroism. Density-functional theory (DFT) calculations show that the stability of MEC films is significantly higher than that of high-index Cu surfaces. Through a combination of high-throughput DFT calculations and theoretical modeling, we demonstrate the high enantioselectivity (42% e.e.) of the chiral MEC for serine, a prototype molecule for studying enantioselective adsorption. The QCM results show that the adsorption amount of L-serine is 1.58 times higher than that of D-serine within a concentration range of 0-60 mM. These findings demonstrate the potential application of MECs in chiral recognition.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Chiral Recognition on Bare Gold Surfaces by Quartz Crystal Microbalance.Angew Chem Int Ed Engl. 2021 Nov 15;60(47):25028-25033. doi: 10.1002/anie.202110187. Epub 2021 Oct 21. Angew Chem Int Ed Engl. 2021. PMID: 34545674
-
Chiral zinc oxide functionalized quartz crystal microbalance sensor for enantioselective recognition of amino acids.Talanta. 2023 Jul 1;259:124496. doi: 10.1016/j.talanta.2023.124496. Epub 2023 Apr 5. Talanta. 2023. PMID: 37031543
-
Chiral thin films of metal oxide.Chemistry. 2013 Jul 29;19(31):10295-301. doi: 10.1002/chem.201300760. Epub 2013 Jun 21. Chemistry. 2013. PMID: 23794366
-
Nanophotonic Platforms for Chiral Sensing and Separation.Acc Chem Res. 2020 Mar 17;53(3):588-598. doi: 10.1021/acs.accounts.9b00460. Epub 2020 Jan 8. Acc Chem Res. 2020. PMID: 31913015 Review.
-
Applications of homochiral metal-organic frameworks in enantioselective adsorption and chromatography separation.Electrophoresis. 2014 Oct;35(19):2733-43. doi: 10.1002/elps.201400079. Epub 2014 May 19. Electrophoresis. 2014. PMID: 24658972 Review.
References
-
- Gong, W., Chen, Z., Dong, J., Liu, Y. & Cui, Y. Chiral metal–organic frameworks. Chem. Rev.122, 9078–9144 (2022). - PubMed
-
- Sallembien, Q., Bouteiller, L., Crassous, J. & Raynal, M. Possible chemical and physical scenarios towards biological homochirality. Chem. Soc. Rev.51, 3436–3476 (2022). - PubMed
-
- Smith, S. W. Chiral Toxicology: It’s the same thing…only different. Toxicol. Sci.110, 4–30 (2009). - PubMed
-
- Shukla, N. & Gellman, A. J. Chiral metal surfaces for enantioselective processes. Nat. Mater.19, 939–945 (2020). - PubMed
-
- Wang, Y., Yang, S., Fuentes-Cabrera, M., Li, S. & Liu, W. Enhancing enantiomeric separation with strain: The case of serine on Cu(531). J. Am. Chem. Soc.139, 8167–8173 (2017). - PubMed
Grants and funding
- SKL202402014/Department of Science and Technology of Jilin Province (Jilin Province Science and Technology Department)
- 22173047/National Natural Science Foundation of China (National Science Foundation of China)
- Grant M-0147/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

