Functional specificity of liquid-liquid phase separation at the synapse
- PMID: 39572583
- PMCID: PMC11582584
- DOI: 10.1038/s41467-024-54423-7
Functional specificity of liquid-liquid phase separation at the synapse
Abstract
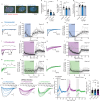
The mechanisms that enable synapses to achieve temporally and spatially precise signaling at nano-scale while being fluid with the cytosol are poorly understood. Liquid-liquid phase separation (LLPS) is emerging as a key principle governing subcellular organization; however, the impact of synaptic LLPS on neurotransmission is unclear. Here, using rat primary hippocampal cultures, we show that robust disruption of neuronal LLPS with aliphatic alcohols severely dysregulates action potential-dependent neurotransmission, while spontaneous neurotransmission persists. Synaptic LLPS maintains synaptic vesicle pool clustering and recycling as well as the precise organization of active zone RIM1/2 and Munc13 nanoclusters, thus supporting fast evoked Ca2+-dependent release. These results indicate although LLPS is necessary within the nanodomain of the synapse, the disruption of this nano-organization largely spares spontaneous neurotransmission. Therefore, properties of in vitro micron sized liquid condensates translate to the nano-structure of the synapse in a functionally specific manner regulating action potential-evoked release.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

