Microbial composition of gastric lesions: differences based on Helicobacter pylori virulence profile
- PMID: 39572621
- PMCID: PMC11582621
- DOI: 10.1038/s41598-024-80394-2
Microbial composition of gastric lesions: differences based on Helicobacter pylori virulence profile
Abstract
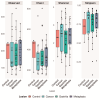
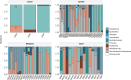
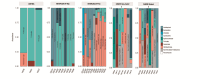
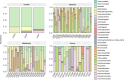
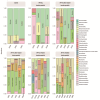
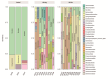
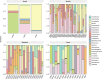
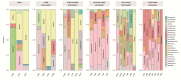
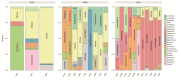
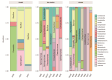
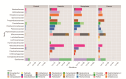
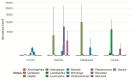
Helicobacter pylori infection is a major risk factor for gastric adenocarcinomas. In the case of the intestinal subtype, chronic gastritis and intestinal metaplasia are well-known sequential steps in carcinogenesis. H. pylori has high genetic diversity that can modulate virulence and pathogenicity in the human host as a cag Pathogenicity Island (cagPAI). However, bacterial gene combinations do not always explain the clinical presentation of the disease, indicating that other factors associated with H. pylori may play a role in the development of gastric disease. In this context, we characterized the microbial composition of patients with chronic gastritis (inactive and active), intestinal metaplasia, and gastric cancer as well as their potential association with H. pylori. To this end, 16 S rRNA metagenomic analysis was performed on gastric mucosa samples from patients with different types of lesions and normal gastric tissues. Our main finding was that H. pylori virulence status can contribute to significant differences in the constitution of the gastric microbiota between the sequential steps of the carcinogenesis cascade. Differential microbiota was observed in inactive and active gastritis dependent of the H. pylori presence and status (p = 0.000575). Pseudomonades, the most abundant order in the gastritis, was associated the presence of non-virulent H. pylori in the active gastritis. Notably, there are indicator genera according to H. pylori status that are poorly associated with diseases and provide additional evidence that the microbiota, in addition to H. pylori, is relevant to gastric carcinogenesis.
Keywords: Gastric cancer; Helicobacter pylori; Metagenomics.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: The study was approved by Ethics Committee (COMEP) of the Federal University of Ceará (protocol 071002/10) and conducted in accordance with the Declaration of Helsinki. All participants of the study signed individual informed consent forms.
Figures
References
-
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin.71(3), 209–249. 10.3322/caac.21660 (2021). - PubMed
-
- Oue, N., Sentani, K., Sakamoto, N., Uraoka, N. & Yasui, W. Molecular carcinogenesis of gastric cancer: Lauren classification, mucin phenotype expression, and cancer stem cells. Int J Clin Oncol.24(7), 771–778. 10.1007/s10147-019-01443-9 (2019). - PubMed
-
- Conteduca, V. et al. pylori infection and gastric cancer: state of the art (review). Int J Oncol.42(1), 5–18. 10.3892/ijo.2012.1701 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

