Tau filaments are tethered within brain extracellular vesicles in Alzheimer's disease
- PMID: 39572740
- PMCID: PMC11706778
- DOI: 10.1038/s41593-024-01801-5
Tau filaments are tethered within brain extracellular vesicles in Alzheimer's disease
Abstract
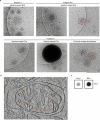
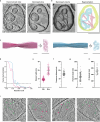
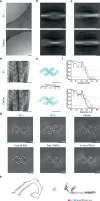
The abnormal assembly of tau protein in neurons is a pathological hallmark of multiple neurodegenerative diseases, including Alzheimer's disease (AD). Assembled tau associates with extracellular vesicles (EVs) in the central nervous system of individuals with AD, which is linked to its clearance and prion-like propagation. However, the identities of the assembled tau species and EVs, as well as how they associate, are not known. Here, we combined quantitative mass spectrometry, cryo-electron tomography and single-particle cryo-electron microscopy to study brain EVs from individuals with AD. We found tau filaments composed mainly of truncated tau that were enclosed within EVs enriched in endo-lysosomal proteins. We observed multiple filament interactions, including with molecules that tethered filaments to the EV limiting membrane, suggesting selective packaging. Our findings will guide studies into the molecular mechanisms of EV-mediated secretion of assembled tau and inform the targeting of EV-associated tau as potential therapeutic and biomarker strategies for AD.
© 2024. Crown.
Conflict of interest statement
Competing interests: K.E.D. is a board member of Ceracuity, Inc. The remaining authors have no conflicts of interest to declare.
Figures






Update of
-
Tau filaments are tethered within brain extracellular vesicles in Alzheimer's disease.bioRxiv [Preprint]. 2023 Apr 30:2023.04.30.537820. doi: 10.1101/2023.04.30.537820. bioRxiv. 2023. Update in: Nat Neurosci. 2025 Jan;28(1):40-48. doi: 10.1038/s41593-024-01801-5. PMID: 37163117 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- AARF-22-923826/ALZ/Alzheimer's Association/United States
- AG056151/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- AG017617/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- A2017393S/BrightFocus Foundation (BrightFocus)
- P01 AG017617/AG/NIA NIH HHS/United States
- AG056732/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- AG063521/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- (MC_UP_1201/25/RCUK | Medical Research Council (MRC)
- RF1 AG056151/AG/NIA NIH HHS/United States
- RF1 AG063521/AG/NIA NIH HHS/United States
- RF1 AG057517/AG/NIA NIH HHS/United States
- R01 AG056732/AG/NIA NIH HHS/United States
- ARUK-RS2019-001/Alzheimer's Research UK (ARUK)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

