Preferential graphitic-nitrogen formation in pyridine-extended graphene nanoribbons
- PMID: 39572756
- PMCID: PMC11582605
- DOI: 10.1038/s42004-024-01344-7
Preferential graphitic-nitrogen formation in pyridine-extended graphene nanoribbons
Abstract
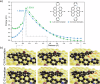
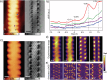
Graphene nanoribbons (GNRs), nanometer-wide strips of graphene, have garnered significant attention due to their tunable electronic and magnetic properties arising from quantum confinement. A promising approach to manipulate their electronic characteristics involves substituting carbon with heteroatoms, such as nitrogen, with different effects predicted depending on their position. In this study, we present the extension of the edges of 7-atom-wide armchair graphene nanoribbons (7-AGNRs) with pyridine rings, achieved on a Au(111) surface via on-surface synthesis. High-resolution structural characterization confirms the targeted structure, showcasing the predominant formation of carbon-nitrogen (C-N) bonds (over 90% of the units) during growth. This favored bond formation pathway is elucidated and confirmed through density functional theory (DFT) simulations. Furthermore, an analysis of the electronic properties reveals metallic behavior due to charge transfer to the Au(111) substrate accompanied by the presence of nitrogen-localized states. Our results underscore the successful formation of C-N bonds on the metal surface, providing insights for designing new GNRs that incorporate substitutional nitrogen atoms to precisely control their electronic properties.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interest.
Figures





References
-
- Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature466, 470–473 (2010). - PubMed
-
- Talirz, L. et al. On-surface synthesis and characterization of 9-atom wide armchair graphene nanoribbons. ACS Nano11, 1380–1388 (2017). - PubMed
-
- Yamaguchi, J. et al. Small bandgap in atomically precise 17-atom-wide armchair-edged graphene nanoribbons. Commun. Mater.1, 36 (2020).
-
- Chen, Y.-C. et al. Tuning the band gap of graphene nanoribbons synthesized from molecular precursors. ACS Nano7, 6123–6128 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

