Effect of Cordyceps militaris extract containing cordycepin on the adipogenesis and lipolysis of adipocytes
- PMID: 39572891
- PMCID: PMC11788751
- DOI: 10.1002/2211-5463.13930
Effect of Cordyceps militaris extract containing cordycepin on the adipogenesis and lipolysis of adipocytes
Abstract
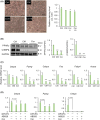
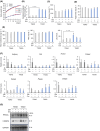
Obesity, a global health concern, results from an energy imbalance leading to lipid accumulation. In the present study, Cordyceps militaris extract (CM) and its primary component, cordycepin, were investigated to characterize their potential effects on adipogenesis and lipolysis. Treatment with CM or cordycepin reduced lipid droplets and increased hormone-sensitive lipase activation in 3T3-L1 cells. In a diabetic obese mouse model, CM and cordycepin lowered serum low-density lipoprotein/very low-density lipoprotein levels and reduced oxidative stress and cell senescence markers. Thus, cordycepin inhibits preadipocyte differentiation and promotes lipolysis, which may serve as a novel obesity treatment. Further studies, including clinical trials, are required to validate the clinical potential of cordycepin.
Keywords: Cordyceps militaris; adipocyte; cell senescence; cordycepin; oxidative stress.
© 2024 The Author(s). FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

