Combined Photopolymerization and Localized Photochromism by Aza-Diarylethene and Hemiindigo Synergy
- PMID: 39573834
- PMCID: PMC11756035
- DOI: 10.1002/adma.202411223
Combined Photopolymerization and Localized Photochromism by Aza-Diarylethene and Hemiindigo Synergy
Abstract
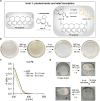
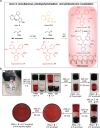
Molecular photoswitches produce light-controlled changes at the nanometer scale and can therefore be used to alter the states and behavior of materials in a truly bottom-up fashion. Here an escalating photonic complexity of material property control with light is shown using a recently developed aza-diarylethene in combination with hemiindigo (HI) photoswitches. First, aza-diarylethene can be used as a photoswitch in polystyrene (PS) to reversibly inscribe relief-type 3D structures into PS. Second, aza-diarylethene can further be used as a photoinitiator for light-induced polymerization of methyl acrylate (MA), demonstrating for the first time light-controlled chemical reactivity control with its zwitterionic switching state. Third, aza-diarylethene and HIs are implemented into aza-diarylethene polymerized MA, generating photochromic polymers. At the fourth level, a binary mixture allows to synergize aza-diarylethene-induced photopolymerization with localized photochromism changes of the simultaneously entrapped functional HI. With such multilevel light response, the utility of this particular photoswitch combination for applications in advanced photonic materials is demonstrated.
Keywords: aza‐diarylethene; hemiindigo; light‐responsive materials; photochromism; photopolymerization.
© 2024 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Feringa B. L., Browne W. R., Molecular Switches, 2nd ed., Wiley‐VCH, Weinheim, Germany: 2011.
-
- Pianowski Z. L., Molecular Photoswitches. Chemistry,Properties, and Applications, Wiley‐VCH, Weinheim, Germany: 2022.
-
- Goulet‐Hanssens A., Eisenreich F., Hecht S., Adv. Mater. 2020, 32, 1905966. - PubMed
-
- Boelke J., Hecht S., Adv. Opt. Mater. 2019, 7, 1900404.
-
- Lu P., Ahn D., Yunis R., Delafresnaye L., Corrigan N., Boyer C., Barner‐Kowollik C., Page Z. A., Matter 2021, 4, 2172.
Grants and funding
LinkOut - more resources
Full Text Sources

