Trametinib alters contractility of paediatric Noonan syndrome-associated hypertrophic myocardial tissue slices
- PMID: 39573843
- PMCID: PMC12055358
- DOI: 10.1002/ehf2.15173
Trametinib alters contractility of paediatric Noonan syndrome-associated hypertrophic myocardial tissue slices
Abstract
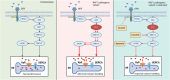
Aims: No curative treatment is available for RASopathy-associated childhood-onset hypertrophic cardiomyopathy (RAS-CM). Preclinical data and individual reports suggest a beneficial effect of small molecules targeting the RAS-mitogen-activated protein (MAP) kinase (MAPK) pathway in severely affected RAS-CM patients. The aim of this study was to evaluate the biophysical effects of trametinib, rapamycin and dasatinib on cultivated myocardial tissue slices of a paediatric RAS-CM patient using biomimetic cultivation chambers (BMCCs) and to correlate the findings with clinical data.
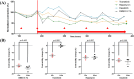
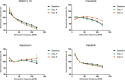
Methods: Contracting right ventricular (RV) tissue slices were prepared from resected myocardium, cultivated in BMCCs and treated with distinct molecules directly and indirectly targeting the RAS-MAPK pathway (trametinib, rapamycin and dasatinib) or dimethyl sulfoxide (DMSO). Tissue biophysical properties were assessed using electrical stimulation protocols. Contractile function, force-frequency relationship and post-pause potentiation were compared before and after treatment. These parameters correlated to L-type Ca2+ channel function and sarcoplasmic Ca2+ loading.
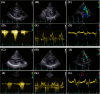
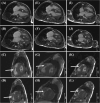
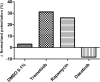
Results: In vivo, off-label treatment with MAPK kinase (MEK) inhibitor trametinib of a child with severe RAS-CM resulted in a modest reduction of RV outflow tract (RVOT) obstruction (RVOT 151 to 122 mmHg after 11 weeks) and improved diastolic function (E/A 0.68 to 1.09 after 11 weeks) and myocardial strain [RV global radial strain (RV-GRS) 25.94 to 42.76; RV global circumferential strain (RV-GCS) -15.26 to -18.61; and RV global longitudinal strain (RV-GLS) -10.31 to -16.78 at 11 weeks], as determined by echocardiography and cardiac magnetic resonance tomography. In cultivated RV myocardial tissue slices, contraction force decreased after addition of trametinib and rapamycin but not after addition of DMSO and dasatinib. Improvement of Ca2+ handling, as depicted by a more positive force-frequency relationship and enhanced post-pause potentiation (31.2%), was noted in the trametinib-treated slice. The increase in post-pause potentiation was less pronounced in rapamycin-treated (26%) and absent in dasatinib-treated (<1%) slices.
Conclusions: Ex vivo analysis of cultivated and electrically stimulated RV myocardial tissue slices of a patient with RAS-CM showed decreased contractility and improved sarcoplasmic reticulum function after addition of trametinib and in part after addition of rapamycin, but not after addition of dasatinib.
Keywords: Noonan; RAF1; cultivation; myocardial slices; trametinib.
© 2024 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
AD is a shareholder of InVitroSys GmbH, which developed the MyoDish cultivation system used for this study. CMW is a consultant for Bristol Myers Squibb, Rocket Pharmaceuticals, Day One Biopharmaceuticals, BioMarin Pharmaceutical, Adrenomed AG and Pliant Therapeutics. CMW has an ownership interest in Preventage Therapeutics.
Figures

References
-
- Lipshultz SE, Orav EJ, Wilkinson JD, Towbin JA, Messere JE, Lowe AM, et al. Risk stratification at diagnosis for children with hypertrophic cardiomyopathy: an analysis of data from the Pediatric Cardiomyopathy Registry. Lancet 2013;382:1889‐1897. doi: 10.1016/S0140-6736(13)61685-2 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
